Chapter Two
The search for acute and chronic health effects from uranium exposure dates to 1824 when Christian Gmelin reported on uranyl nitrate, chloride, and sulphate in dogs and rabbits (Hodge, 1973). Early studies of humans were conducted from about 1860 into the early 1900s, during which time uranium was administered as a therapeutic agent for diabetes because it had been shown to increase glucose excretion (Hodge, 1973). The Manhattan Project (1943-1953) and atomic energy program served to increase vastly the collective knowledge of the biological consequences of exposure to uranium and uranium compounds. However, during these incipient years of research, the work pertained to natural uranium and its compounds and, accordingly, dealt with the chemical toxicity of uranium (USAEC, 1958). This early period of research on the chemistry and toxicology of uranium produced extensive reviews (Hodge, 1973; Tannenbaum, 1951; Voegtlin and Hodge, 1949). The radiological toxicity of enriched uranium was studied much later (Leach, 1970, 1973; Filippova, 1978; Bair, 1970). Very little health-related research has been completed specifically on DU, but there has been extensive research on natural and enriched uranium, both of which pose a greater radiological hazard and an identical toxicological chemical hazard.
Health effects of DU are determined by many factors. One of the first factors to consider is whether the DU exposure was internal or external. Health effects related to internal exposure may result from either chemical or radiological toxicity but depend on the extent of exposure. Three main pathways exist by which internalization of uranium may occur: ingestion, inhalation, and embedded fragments or wound contamination. The characteristic physicochemical properties of uranium metal and each uranium compound influence its uptake and distribution in various pools or compartments in the body and, eventually, its elimination by the body and, therefore, the resulting chemical and radiological toxicity. In contrast to internal health effects, the possible health effects related to external exposure are limited to radiological exposure. The balance of the report is structured to deal with the health effects of internal and external exposure to DU in a military environment.
In nonmilitary situations the main routes of uranium uptake by the human body are inhalation and ingestion, as is the case with other heavy metals. In the military environment, additional routes of uranium exposure exist such as from embedded metal fragments slowly dissolving in the body and uranium-contaminated open wounds. All of these routes of internalization contribute to the total body burden of uranium.
Outside the military or industrial settings, the major portion of the natural body burden of uranium for typical civilians is derived from ingested and inhaled material. There is a limited amount of natural uranium in the air we breathe, the food we eat, and water we drink. The origin of the uranium may be natural, or it may have been contributed to the environment by man-made sources, such as application of uranium-containing superphosphate fertilizer to crop land or combustion of fossil fuel.
Once uranium enters the body, a portion will be soluble as determined by its chemical and physical characteristics. The more soluble a compound is, the quicker it will be absorbed from the lung into the blood and the more completely it will be absorbed from lung and gastrointestinal tract; fractional absorption of uranium compounds from the intestine is generally low. The most-soluble compounds will be absorbed from the lungs within hours or days (Type F for fast dissolution, formerly called Class D). Occupationally, workers processing uranium are usually exposed to its more soluble forms. These include uranyl fluoride, uranium tetrachloride, and other nonoxide compounds. The less-soluble compounds are more likely to require weeks to be solubilized and absorbed (Type M for medium dissolution, formerly called Class W). These compounds include UO3 as well as nonoxide compounds. The relatively insoluble compounds are more likely to require years to be solubilized and absorbed (Type S for slow dissolution, formerly called Class Y). These compounds include UO2 and U3O8 (ICRP, 1995). Even the metallic form slowly solubilizes in biological fluids. Natural environmental exposure includes both soluble and insoluble forms of uranium.
The solubility determines the ease of absorption and, therefore, the chemical dose delivered to the target organ and its chemical toxicity. In general, the soluble compounds, such as the halides and uranates, are far more toxic to the kidney because they promote a greater blood concentration, while insoluble compounds, such as oxides are more toxic to the lung because their longer residence in the lung produces a larger radiation dose. (Domingo, 1987; Maynard and Hodge, 1949; Stokinger, 1981). As previously discussed, many of these compounds are laboratory reagents and industrial chemicals and are absent from the military environment. As such, they are not relevant to the discussion of health effects related to the military use of DU.
Uranium dissolved in blood circulates bound to erythrocytes, as a complex with transferrin and other plasma proteins, and as diffusible complexes with low molecular weight, ligands (Voegtlin and Hodge, 1949; Hursh and Spoor, 1973). Diffusibility implies also that these compounds are filtered in the glomerulus. The carbonate-bicarbonate complexes are of special importance, but it is likely that complexes with glutathione and other sulfhydryl compounds also exist (Voegtlin and Hodge, 1949; Hursh and Spoor, 1973).
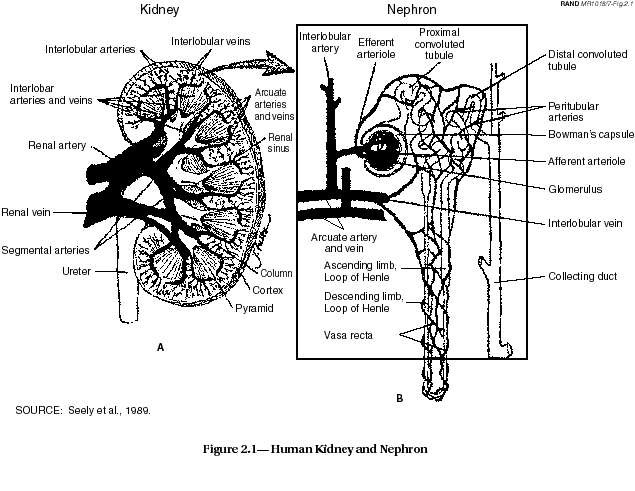
The kidney (see Figure 2.1) is considered the target organ for uranium for chemical toxicity. The primary renal site of action of uranium is the proximal tubule where proton secretion degrades the bicarbonate complex of the uranyl ion, permitting the uranium to react with apical cell membranes of the tubular epithelium. This view is supported by the observation that alkalinization of urine increases urinary uranium excretion. It has not been established whether the reaction between uranyl ions and the cell membrane directly interferes with membrane function, as has been reported in yeast, or whether the primary target of the metal lies inside the renal cell.
Like mercury, cadmium, and other heavy-metal ions, excess uranyl ions depress glomerular function, tubular secretion of organic anions, and reabsorption of filtered glucose and amino acids in the proximal tubule, and the function of the more distal nephron segments (Bowman and Foulkes, 1970). The biological oxidation of uranium compounds to the divalent uranyl cation of hexavalent uranium has already been mentioned. The uranyl ion, in turn, forms a variety of salts and biological complexes, chief among which are the carbonate compounds.
Although there is no assurance that the toxicity of uranium is the same in various tissue compartments, the overall maximum permissible (renal) concentration (MPC) has frequently been suggested to be 3 �g uranium/g kidney (Voegtlin and Hodge, 1949; Hursh and Spoor, 1973) (See Chapter One, "Recommended Kidney Concentration," p. 12, for more information). The MPC is defined here as that metal concentration in the kidney associated with a significant increase of the frequency of kidney malfunction, such as proteinuria and glucosuria. However, limited confidence can be attributed to the significance of that figure for the general population for several reasons.[1] In fact, it is difficult to define a unique MPC of uranium in the kidney that will be valid for all types of exposure of differing populations. In spite of the incomplete justification for a unique MPC of uranium in the kidney, existing guidelines based on the figure of 3 �g per gram kidney have appeared to protect at-risk human populations adequately (see below, "Inhalation--Chemical Toxicity").
The schematic in Figure 2.2 depicts how uranium interacts with the body. Inhaled, ingested, or embedded fragments reach the blood after solubilizing either at the site of entry or at some other location in the body where they end up. For instance, some inhaled uranium enters the blood from the lungs, and some of the uranium originally in the lungs ends up in the gastrointestinal tract as a result of mucociliary clearance from the respiratory tract and subsequent swallowing. Uranium then accumulates to some degree in all organs. As discussed, the major portion of uranium in blood is excreted in the urine, with the remainder distributed mostly to bone and soft tissue.[2] There are few data to show the content and distribution of uranium in human tissue from inhalation and ingestion from natural sources. Fisenne et al. (1988, 1993) summarized all of the published data for uranium in human tissue and blood. The measurements are shown in Table 2.1. The normal range for the total mass of uranium in a human being is 2-62 �g (ERDA, 1975; USUR, 1984; Wrenn, 1985; Fisenne, 1986; Fisenne, 1994).
Figure 2.2--Compartmental Model for Internalization of Depleted Uranium
Bone ash data provide an insight into the amount of natural uranium inhaled and ingested in various countries because in a steady state (see Figure 2.2) the percentage going to bones is known. In spite of large differences across countries, we do not observe known adverse health consequences as a result of these differences. Because the radiological effects of DU are less than those of natural uranium and the chemical effects are identical, we can infer that exposure to DU at these levels would also have few health effects on a population.
The large variation in skeletal uranium content in the United States and other countries demonstrates the difference in daily uptake, mainly from drinking water. This suggests that baseline urinary excretion in the normal population varies widely, and excretion data for exposed persons should be interpreted against a suitable control group.
Table 2.1
Uranium Concentration in Human Tissues and Blood
| mg U per kg Wet Tissue or Blood | |||||||
| Country/Locale | References | Lung | Liver | Kidney | Muscle | Fat | Blood |
| Australia: 5 cities |
Fisenne et al., 1984 |
||||||
| Austria: Unknown |
Hoffman, 1942 |
5 |
0.23�0.18 |
||||
| Brazil: Sao Paulo |
Fisenne and Perry, 1980 |
||||||
| Canada: 5 cities |
Fisenne and Perry, 1980 |
||||||
| England: London |
Hamilton, 1972 | 0.25 |
0.19 |
0.6 |
0.69�0.45 |
||
| India: Bombay |
Ganguly, 1970; Parshad et al., 1980 |
2.2 |
1.3�0.3 |
||||
| Japan: Tokyo 4 cities Tokyo |
Igarashi et al., 1985 Nozaki et al., 1970 Ikeda et al., 1977 |
170 |
0.24 |
0.34 |
0.43 |
0.64�0.65 |
|
| Nepal: Katmandu |
Fisenne et al., 1984 |
||||||
| Nigeria: Lagos |
Fisenne and Perry, 1980 |
||||||
| USSR: Moscow Moscow Minsk |
Fisenne et al., 1984 Drutman and Mordasheva, 1985 Malenchenko et al., 1972 |
5.4�0.8 6.8�0.5 |
11�1.5 5.8�0.6 |
5.3�0.9 5.5�0.6 |
6.5�1.0 7.7�1.1 |
1.9 |
1.1�0.02 |
| United States: New York Illinois 5 regions Pennsylvania Utah Colorado Wisconsin |
Fisenne and Welford, 1986 Fisenne and Perry, 1985 Lucas, 1983 Broadway, 1983 Singh et al., 1986 Singh et al., 1986 Singh et al., 1986 Fisenne and Perry, 1980 |
0.50�0.39 1.17 1.02 1.02 |
0.20�0.31 0.12 0.33 0.30 |
0.43�0.26 0.39 0.90 1.00 |
0.14�0.09 0.1 |
||
| Yugoslavia: Unknown |
Picer and Strohl, 1968 |
0.45 | |||||
| All: n= | 7 | 8 | 9 | 4 | 2 | 8 | |
| Mean + SD | 2.5�2.5 | 2.3�4.0 | 2.3�2.3 | 3.7�4.0 | 1.3�0.9 | 0.58�0.44 | |
| Range | 0.50-6.8 | 0.12-11 | 0.34-5.5 | 0.19-7.7 | 0.6-1.9 | 0.1-1.3 | |
Table 2.1--continued
| Ratio | |||||||
| Country/Locale | Bone | Bone Type | Bone Ash (�g U/kg) |
MMb | Bone/ Blood |
Liver/ Kidney |
|
| Australia: 5 cities |
0.49�0.36 |
FRV (8 ) |
3.5�2.6 |
3 |
|||
| Austria: Unknown |
0.53 |
F(1) |
1.5 |
1 |
2.30 |
||
| Brazil: Sao Paulo |
7.7�1.1 |
F(2) |
22�3 |
3 |
|||
| Canada: 5 cities |
2.5�2.0 |
V(10) |
18�14 |
3 |
|||
| England: London |
3.5�0.9 |
6 types (61) |
23�6 |
2 |
5.07 |
||
| India: Bombay |
5.3 |
?(ng) |
21 |
2 |
4.08 |
||
| Japan: Tokyo 4 cities Tokyo |
0.75�0.29 2.2�0.4 2.7�1.9 |
FRS(17) RS1(46) FMRS(21) |
2.5�1.3 8.6�1.4 8.8�9.1 |
2 4 2 |
4.22 |
1.42 |
|
| Nepal: Katmandu |
2.2�1.0 |
V(9) |
16�7 |
3 |
|||
| Nigeria: Lagos |
7.1�0.6 |
V(3) |
51�4 |
3 |
|||
| USSR: Moscow Moscow Minsk |
2.7�0.1 3.6�1.8 5.5�2.0 |
5 types (2) 5 types (152) RS(30) |
11�1 15�5 18�7 |
3 1 1 |
3.27 |
0.48 0.95 |
|
| United States: New York Illinois 5 regions Pennsylvania Utah Colorado Wisconsin |
0.33�0.20 0.25�0.30 0.82�0.60 0.39 0.67 0.61 1.7 0.92 1.9 1.2�0.3 |
V(58) ?(33) V(10) V(13) R(13) V(53) R(53) V(19) R(19) Sk(6) |
2.4�1.4 1.7�2.0 5.5�4.0 2.6 2.7 4.0 6.8 6.1 7.7 4.9�1.1 |
1 2 3 3 3 3 3 3 3 3 |
2.36 2.50 |
2.15 3.25 2.73 3.33 |
|
| Yugoslavia: Unknown |
0.10 |
?(ng) |
0.41 |
4 |
0.22 |
||
| All: n= | 26 | 26 | |||||
| Mean + SD | 2.4�2.3 | 11�11 | |||||
| Range | 0.10-7.1 | 0.4-51 | |||||
a
Bone type and number of measurements: F = femur, M = mandible, R = rib, S = skull, Sk = blended skeletons, St = sternum, V = vertebrae, ? = unspecified. The number of measurements is given in parentheses; ng = not given.bMM = Measurement method: 1 = fluorimetry; 2 = fission track; 3 = alpha spectrometry; 4 = neutron activation analysis; 5 = gamma spectrometry. The percentage of dry ash used to convert values given in mass or activity per kg wet weight for the various bone types were sternum and vertebrae = 5%; rib = 25%; femur, mandible, and skull = 35%; unspecified = 25%. The standard deviations noted in the table were either given by the author(s) or calculated from the published data. In some cases only a mean value without a standard deviation is given.
A plot of the data for uranium in bone ash for the 12 countries is shown in Figure 2.3. The bone ash data fit a log-normal distribution better than a normal distribution. The geometric mean is 7.3 �g/kg ash (88 mBq 238U/kg ash), with a geometric standard deviation of 4.2, indicating a very skewed distribution. This spread is related to dietary habits and to the water concentration in particular. The arithmetic mean of bone ash data is 11 � 11 �g/kg ash (130 mBq 238U/kg ash) with a range of 0.4 to 51 �g/kg ash (5 to 610 mBq 238U/kg ash). In short, bone ash data provide an insight into the amount of uranium that naturally distributes in the bone in various countries. We find a 100-fold difference across countries, with concentrations increasing with age. These data show that what is high in one area may be normal in another but that areas with higher rates do not have known resulting adverse health consequences. Given that depletion of uranium does not alter its chemical properties, and that radiation from DU is less than from natural uranium, exposure to DU at these levels would appear not to have known adverse health consequences.
Uranium was reported in tissue samples of lung, kidney, skeleton, and liver from an urban population in Bombay, India (Dang et al., 1995). The measurement was by neutron activation and gamma ray counting of the Neptunium-239 (239Np) fission product. The arithmetic and geometric mean organ burdens reported are shown in Table 2.2. The reported concentration for 15 samples of skeleton tissue is 0.56 �g U/kg wet weight (7 mBq 238U/kg wet weight), with a range of 0.1 to 1.6 �g U/kg wet weight (1 to 19 mBq 238U/kg wet weight).
To compare the skeletal value with the data for Bombay in Table 2.2, the wet weight is about 2 �g U/kg ash weight (25 mBq 238U/kg ash), or a factor of ten below the Bombay value reported earlier. The reason for this is not known. It suggests that given the skewed nature of uranium distribution in bone large numbers of samples are needed to provide a reliable estimate of the mean value. Clinically, uranium exists in small amounts in all organs, and no radiological or toxicological effect is observed. Note that the concentration here is far below the 3 �g U/g of kidney de facto standard.
Table 2.2
The Concentration of Uranium in Different Human Tissues in an Urban Population in
Bombay, India, Compared with Literature Values (in �g U/kg wet weight)
| Tissue | Literature Rangea | Mean (Bombay) | SD (Bombay) | Geometric Mean (Bombay) | Geometric Standard Deviation (Bombay) |
| Lungs | 0.34-3.60 (15)b | 1.45 | 1.00 | 1.13 | 2.0 |
| Kidneys | 0.11-2.54 (15) | 0.81 | 0.76 | 0.57 | 2.6 |
| Skeleton | 0.10-1.63 (15) | 0.56 | 0.45 | 0.40 | 2.4 |
| Liver | 0.016-0.34 (15) | 0.12 | 0.11 | 0.08 | 3.3 |
| Heart | 0.07-0.40 (12) | 0.18 | 0.12 | 0.19 | 2.6 |
| Muscle | 0.003-0.22 (15) | 0.09 | 0.07 | 0.06 | 2.7 |
SOURCE: Dang et al., 1995.
aLiterature values from Fisenne, 1986; Igarashi, 1985; Hamilton, 1972; Wrenn, 1985.
bNumber of samples is in parentheses.
A measurement of the uranium concentration of 83 �g U/kg bone ash (1,000 mBq 238U/kg ash) from Beijing residents was reported by Lianquing (1988). This point, if added to the original published data in Table 2.1, would be the highest reported concentration in bone ash to date. The water in Beijing is also elevated in 238U, with intake estimated at 110 mBq/day.[3]
One study was performed on tissue samples separated by age to investigate whether there is an age dependence of uranium in the available organs (Fisenne, 1986) (see Figure 2.4). There were 27 lung, 58 vertebra, 27 liver, and 12 kidney samples from New York City accident victims, ranging in age from 14 to 73 years. The data were combined into six age groups. The results are shown in Table 2.4. There was no statistically significant difference due to sex. The analysis of variance of the uranium concentration in lung and vertebrae showed an increase in the means with age at the 95 percent confidence level. There was a threefold increase in lung concentration and twofold increase in the vertebra concentration over the age range. The relatively large increase in lung concentration with age probably indicates that the inhalation intake is retained in the pulmonary lymph nodes for long periods (Fisenne, 1986). There is no statistically significant difference in uranium concentration among bone types (i.e., vertebra, rib, femur) (Harley, 1990).
Figure 2.5 shows the average distribution of 238U in the body from chronic intake, calculated from Table 2.1 and standard organ weights (Fisenne, 1993). The organ contents in decreasing amount are skeleton (380 mBq), muscle (132 mBq), fat (110 mBq), blood (25 mBq), lungs (12 mBq), liver (5.4 mBq), and kidneys (2.4 mBq). The total body content is 670 mBq (56 �g).
Several authors note that the log normal distribution of uranium in bone seen in Figure 2.3, with a range of over a factor of ten, does not agree with the reported range of dietary intake of only a factor of three. The reason for this is not known but probably represents the influence of water on the total uranium intake.
Following exposure to uranium and its distribution among various body compartments, or pools, uranium disappears from various tissues at different rates. The half-life in bone is approximately 300 days while in blood it is less than one-half day (Hursh and Spoor, 1973). In the lungs (Eidson, 1994), retention of uranium depends on the solubility of the various oxides to which the lung is initially exposed. Even within some tissues, the uranium is distributed between compartments and exhibits a range of half-lives. Renal uranium, for instance, is lost at rates reflecting its presence in at least two distinct kinetic pools, one with a relatively short half-life of around six days and a second one with an indeterminate half-life (Hursh and Spoor, 1973). This conclusion is complicated by the likelihood that, as with other metals, the onset of renal damage will decrease the retention time of uranium.
Uranium is present in limited concentrations in the air we breathe. The origin of this uranium may be natural, or it may result from such human activities as the combustion of fossil fuel. Background inhalation was estimated from measurements in filtered air samples in New York City (Fisenne, 1987).[4] Annual inhalation intake of natural uranium totaled about 14.7 mBq/year (Fisenne, 1987).[5] This agrees well with the average age-weighted annual intake of 14.2 mBq/year reported in UNSCEAR (UNSCEAR, 1993). (See Appendix F for UNSCEAR intake tables.)
Very little of the amount of natural uranium that is inhaled eventually reaches the kidney. This is because the body is amazingly efficient at clearing alien substances through a variety of mechanisms. Of the natural uranium inhaled, 75 percent is exhaled and only 25 percent is retained in the lungs. Of the 25 percent uranium initially retained in the lungs, 80 percent is cleared by the bronchial mucociliary mechanism, which results in most of the natural uranium finding its way to the GI tract where most is excreted and only a fraction enters the bloodstream. Of the 20 percent deposited in the lungs that is not cleared by the mucociliary action, 15 percent ends up in the lymph nodes for a long period and 5 percent enters the blood (either directly from the lungs or from the GI tract or lymph system).[6] Then of the original 100 percent of natural uranium that entered the body via inhalation, less than 1 percent eventually makes its way to the kidney, where it might affect renal function (ICRP, 1995). Figure 2.2 shows a model of the basic features of distribution in the body following inhalation. Figure 2.6 below shows the percentage disposition of inhaled uranium.
In the military environment, DU inhalation exposure may contribute to the total uranium body burden and any health effects that may result from a sufficiently large body burden. When a DU penetrator strikes a hard target, it forms DU dust. From 10 to 35 percent of the original material is aerosolized and approximately 60 to 69 percent of the aerosolized fraction is respirable. From the heat of combustion as well as weathering, these small particles will eventually become oxidized, forming predominately depleted U3O8 but also small amounts of depleted UO2 and depleted UO3 (CHPPM, 1998). Most of the suspended aerosols will rapidly settle to the ground. Activity or surface winds may disturb the settled particles and resuspend and redistribute a fraction of them (see Appendix C).
As discussed, aerosolized particles from the impact are small enough to be inhaled. The primary hazard from inhaled uranium aerosols is related to the extent and rate of transfer of inhaled uranium to the blood and the presumed amounts reaching their primary targets in the kidney. Two factors will influence the degree of hazard: the site of deposition in the respiratory tract, dependent on the aerodynamic equivalent diameter (AED), and the fate in the lung, dependent on the physical and chemical characteristics of the particles, such as the solubility, exposed surface areas, and intercrystalline forces (Eidson, 1994). A radiation dose is delivered to the airways and lung while the uranium remains in the lung.
After inhalation, 95 percent of the larger (greater than 10 �m AED) particles are deposited in the upper respiratory tract (bronchioles, bronchi, trachea). The majority of these particles are cleared to the pharynx by the normal bronchial mucociliary clearance mechanism and swallowed or blown out of the nose. "As such, it is only the smaller respirable particles which represent a potential health hazard from inhaled (natural) uranium. As particle size decreases below 10 �m, deposition decreases in the extrathoracic and bronchial regions but increases in the bronchioles and alveoli (pulmonary regions) such that, at particle sizes below 0.5 �m, the alveoli represent the major site of deposition. Retention of particles in various lung compartments depends on the efficiency of the mucocillary clearance mechanism which decreases in the deeper portions of the lung, or by macrophage action and solubilization." (ICRP 1994.)
The percentage of DU aerosol particles that are respirable varies according to the circumstance by which it oxidizes. For particles generated by fire, the percentage smaller than 10 �m AED ranges from 0.1 to 33, while particles generated from impact of a hard target are virtually all smaller than 10 �m AED (CHPPM, 1998). It is the respirable particles that present a potential health hazard from uranium inhalation.
Respirable DU particles, i.e., particulate DU that reach the alveoli, may be cleared from the lungs by macrophage action or by solution and transfer into the blood. The alveolar epithelium is generally more permeable to dissolved heavy metals than is the intestinal epithelium. This may be related to various factors, such as greater leakiness of the gap junctions between cells, fluidity of cell membranes, relatively long contact time with alveolar tissue, and low concentrations of binding compounds in the alveolar fluids that would make the metal unavailable for absorption (see review by Foulkes, 1994).
Inhalation--Chemical Toxicity. Extensive information is available on the occupational exposure of workers in the uranium industry. These include workers in both uranium mines and in contractor facilities, where uranium was separated from the ore and uranium metal was produced for the enrichment process for nuclear weapons and nuclear fuel. Workers' exposure to uranium during the peak operational era was high in some cases. There were occupational guidelines and standards at the time; however, in many cases these standards were exceeded. Poldenak et al. (1982) report exposures of up to 9,000-10,000 �g natural U/m3 at the Y-12 plant in Oak Ridge and at the Mallinckrodt plant (outside of St. Louis). No adverse health effects appeared in the uranium millers who worked with uranium and were exposed to levels of natural uranium far in excess of present standards.
There is, however, convincing evidence of excess lung cancer in underground uranium miners. It is well established that the carcinogenic agent in the mines is not the uranium ore but the gaseous decay products of radon-222. Radon has two alpha-emitting short-lived decay products that form in the air and deposit efficiently on the bronchial surfaces. Two publications showed that the lung dose from inhaled uranium ore was too low to be of significance relative to the bronchial dose from radon decay products (Harley et al. 1981, 1984).
Considerable effort has been devoted to the follow-up of 11 underground mining cohorts. The risk of lung cancer from 222Rn exposure is now well quantified and provides the data that form the basis for the existing guidelines for indoor radon-222 (NCRP, 1984a, 1984b; NIH, 1994; NRC, 1988; NRC, 1998).
The mines also had other airborne substances, such as ore dust, blast fumes, and diesel fumes (in later years only). The literature indicates that the relationship of lung cancer risk across a broad spectrum of other air contaminants was similar only to exposure to radon-222; no other substance could be implicated (NRC, 1988). Smoking enhanced lung cancer risk in a submultiplicative manner such that for the same radon exposure, the risk of getting cancer among individuals who had ever smoked was twice as high as that of those who never had.
Data relating actual air concentration of uranium and urinary excretion are scant. One large study was conducted by the USAEC Health and Safety Laboratory in a uranium refinery in 1950 and 1951 (Lippman, 1964). Air and urine samples were taken in two plants. In Plant 1, UO2 was hydrofluorinated to UF4 and in Plant 2 the UF4 was fluorinated to UF6. The exposure in Plant 1 was mainly to UO2 and UF4, while in Plant 2 the exposure was to UF6 and its hydrolysis product UO2F2.
Both plants operated 24 hours a day with four shifts of personnel. A urine-sampling program was initiated in 1950, with spot samples taken in pairs, one at the end of the work week and one 48 hours later, just before the men returned to work. The urine-sampling program continued until late 1951 when both plants were permanently closed.
A comprehensive air-sampling program began in 1949 and continued until plant closure. For 1950 and 1951, there are air and urine data for the same group of men. Both general air and breathing zone samples were taken so that time-weighted average air dust exposures could be calculated for each man in the plants. During this period approximately 3,000 air samples and 1,000 urine samples were collected from the operating personnel (Lippman, 1959). The data were analyzed by Lippman (1964) and are shown in Figures 2.7 and 2.8 for both soluble and insoluble uranium.
An excellent review of the uranium excretion data collected by the Health and Safety Laboratory of the USAEC in contractor facilities was prepared by Lippmann et al. (1964). They developed a generalized expression for urinary excretion for any uranium compound as a function of time. The following expression described excretion well:
Excretion rate y = AtB ,
where y = the excretion rate, t = time, and A and B are constants.
No increase in overall deaths has been observed as a result of exposure to uranium in several epidemiological studies of workers exposed to uranium in mills and metal processing plants (Archer, 1973a; Brown, 1987; Checkoway, 1988; Hadjimichael, 1983; Poldenak, 1981; Waxweiler, 1983). In one animal inhalation study examining the effects of UO2, no increased mortality was observed at concentrations of 5 mg UO2/m3 for 5 years (Leach et al. 1970). However, exposure to extraordinarily elevated concentrations of uranium oxides much higher than occupational exposures has been found to induce mortality in animals (Rothstein, 1949; Leach et al., 1984). Rothstein observed increased mortality in a variety of animals exposed to 19 mg UO3/m3 for six hours a day, 5.5 days a week, for four weeks. Leach observed 50 percent mortality when rats were exposed to 8,114 mg U/m3 for 10 minutes. The cause of death, in most cases, was chemically induced renal failure (Leach et al., 1984; HHS, 1998). These exposures are 50 to more than 100,000 times higher than the current maximum permissible concentrations for inhalation exposure to soluble and insoluble uranium compounds, set by the American Conference of Governmental Industrial Hygienists (ACGIH, 1993).
Equivocal evidence was found that associates uranium oxide exposure with hepatic effects. One study examining toxic effects in a variety of animals exposed to concentrations of 19 mg UO3/m3 for four weeks reported moderately fatty livers in some of the rabbits and rats tested, while other animals in the study exhibited no such effect (Rothstein, 1949). In another animal study, animals exposed to 22 mg UO2/m3 for 30 days showed no hepatic effects (Rothstein, 1949). Dygert (1949) found no hepatic effect in animals exposed to concentrations of up to 17 mg U3O8/m3 for 26 days. Likewise, no hepatic effects were observed in animals exposed to 10 mg UO2/m3 for one to two years (Stokinger, 1953).
Hematological effects have been observed in uranium miners exposed to uranium for up to 20 years (ambient uranium concentrations were not noted). Vich and Kriklava (1970) observed small, but statistically significant, differences in hemoglobin concentration, hematocrit values, mean corpuscular volumes, and red blood cell counts. All values were still within normal limits, however, and no damage to red blood cell formation was observed. In another human study, there were no changes in hematological parameters associated with uranium dust (including UO2) at concentrations ranging from 0.5 to 2.5 mg/m3, and for short periods approaching 10 mg/m3, for five years (Eisenbud and Quigley, 1956).
In another study, researchers found that rats exposed to a weighted mean of 19 mg UO3/m3 for four weeks (with short periods approaching 30 to 40 mg UO3/m3) exhibited significant differences in myeloid and lymphoid cells of the bone marrow but found no significant hematological change. No causal association with uranium was inferred (Rothstein, 1949). Several other animal inhalation studies also found no hematological effects. Exposures as high as 22 mg UO2/m3 for 30 days or 17 mg U3O8/m3 for up to 40 days in a variety of animal models found no adverse effects on the blood (Dygert, 1949; Rothstein, 1949; Leach, 1970, 1973).
Immune system effects have not been associated with inhaled uranium oxide exposure in uranium industry workers (Cragle, 1988). In corresponding animal studies, animals exposed to UO2 dusts at a concentration of 5 mg U/m3 for one to five years did not exhibit any pathologic changes in their spleens (Leach, 1970, 1973). Tracheobronchial lymph node fibrosis has been observed and is discussed in the next section, radiation toxicity.
No evidence was found showing an association between uranium inhalation and adverse effects on the nervous system (Kathren, 1986; USNRC, 1986; Zhao, 1990; Brown, 1987; Carpenter, 1988; Cragle, 1988; Poldenak, 1981; Reyes, 1984). There is no information on GI, musculoskeletal, cardiovascular, endocrine, or dermal toxicological effects of uranium oxides (HHS, 1997b). No significant adverse effects on body weight were observed in several animal inhalation studies (Dygert, 1949, Rothstein, 1949; Leach, 1970, 1973).
The issue of possible reproductive and developmental effects from natural uranium and DU is discussed in detail below (see "Reproductive Effects of DU," p. 63).
Uranium has been identified as a nephrotoxic metal in animals, similar to other heavy metals, such as cadmium, lead, and mercury (Goodman, 1995). Indeed, even though a large fraction of the total body burden of uranium following oral uptake may accumulate in the skeleton, the kidney is a major target organ for the metal, and renal shutdown is routinely observed following ingestion or injection of high doses of soluble uranium compounds, such as uranyl salts or ammonium diuranate. Lungs and tracheobronchial lymph nodes have been reported as containing the highest concentrations of uranium following prolonged inhalation exposure to natural uranium dust (mean concentration 5 mg natural UO2/m3, 1 �m mass median particle diameter). Dogs, monkeys, and rats thus exposed for as long as five years showed little evidence of serious injury (Leach et al., 1970). The uranium concentration in the lymph nodes exceeded that in kidneys by several orders of magnitude. Following a two- to six-year post-exposure period, dogs and monkeys showed no evidence of uranium toxicity evidenced in body weight, mortality, or renal histology (Leach et al., 1973). Renal malfunction following uranium inhalation, as further discussed below, has only been observed following much higher exposures.
Because the kidney is the target organ for the chemical effects of uranium, the most dramatic health effects are expected to be associated with the kidneys. Uranium has been identified as a nephrotoxic metal, although less so than cadmium, lead, and mercury (Goodman, 1985). In the nephron, uranium exerts its toxic effect mostly in the proximal tubules but also in the glomerulus and other areas of the tubules (Voegtlin and Hodge, 1949; Hursh and Spoor, 1973). In 1995, the Health Physics Society, on behalf of the American National Standards Institute, issued a Bioassay Program for Uranium. This report (HPS N 13.22-1995) suggests that 40 and 8 mg inhalation intakes of soluble uranium would be expected to behave as threshold intakes for inducing permanent and transient renal injury, respectively. However, many assumptions go into this estimate, e.g., lung deposition based on a default particle size of 1 � AMAD and 50 percent absorption from the lungs (Health Physics Society, 1995). Therefore, values need to be adjusted to suit actual exposure scenarios.
The solubility of the uranium compound to which exposure has occurred affects the intake required to cause kidney damage. Uranium oxides (U3O8 and UO2) are relatively insoluble compounds (Types M and S). As discussed, these uranium compounds are retained longer in the lungs and cause lower toxicity to distal organs such as the kidney than that observed with more soluble industrial compounds. Uranium trioxide is considered between Type F and M (Morrow et al., 1972).
Uranium mill workers occupationally exposed to elevated levels of "yellowcake" have also been studied. Yellowcake can be either ammonium or magnesium diuranate with a mixture of compounds with formal composition that ranges from (NH4)2UO4 to (NH4)2U8O252 with approximate composition for ammonium diuranate of (NH4)2U2O7 (Encyclopedia of Chemical Technology, 1983). The study reported findings that indicate reduced proximal renal tubular reabsorption of amino acids and low molecular weight proteins. Concentration data were not obtained. This finding, including mild proteinuria, aminoaciduria, and a dose-related increase in clearance of �-2 microglobulin relative to that of creatinine, correlated with the duration of uranium exposure (Thun, 1985).
In another study, renal injury was not observed in workers exposed to 0.5 to 2.5 mg/m3 of insoluble uranium dust, including UO2, for about five years (Eisenbud and Quigley, 1956). The authors noted, "The negative findings relative to renal injury among workers exposed to insoluble compounds are particularly significant in view of the high levels of exposure reported."
In animal studies, solubility of the inhaled uranium compound dramatically affects renal outcomes. Although soluble uranium compounds, such as uranium halides, have caused renal damage, insoluble uranium oxides appear far less toxic to the kidneys. A variety of animals exposed to UO2 at a concentration of about 5 mg U/m3 for up to five years did not experience renal damage (Leach et al., 1970, 1973).
Other animal studies did observe nephrotoxic effects. Minimal renal pathology was observed in animals exposed to 10 mg UO2/m3 for up to two years (Stokinger, 1953). Dygert (1949) observed evidence of renal damage as indicated by a moderate degree of regenerative tubular changes in some animals exposed to 17 mg U3O8/m3 for 26 days. In earlier studies examining the toxic effects of uranium trioxide inhalation in a variety of animals, evidence for renal injury was observed only in rabbits at a concentration of 19 mg UO3/m3 for four weeks, or a concentration of 22 mg UO2/m3 for 30 days (Rothstein, 1949).
Human case studies from accidental and chronic exposure as well as animals studied demonstrate regeneration of the damaged tubular epithelium when exposure is discontinued and sometimes, in animals, despite continuation of exposure (Bentley, 1985; Dygert, 1949; Maynard, 1949; Stokinger, 1953, 1981; Rothstein, 1949; Kathren, 1986; Eisenbud and Quigley, 1956). It is not known whether this phenomenon is related to the acquisition of increased tolerance to uranium. The hypothesis that resistance to uranium may be provided by the induction of the metal-binding protein metallothionein, which has been implicated in increased resistance to some other heavy metals, has not been critically tested and supported.
The toxicological significance of results from work on the pulmonary route of uptake of particulate uranium is difficult to evaluate unless the amount of the inhaled material that finds its way into the GI tract can be estimated. The conclusion is justified, however, that relatively short-term exposure to uranium oxide particles at concentrations up to 10 mg per cubic meter of inspired air does not cause renal lesions.
The U.S. Transuranium Registry (Kathren, 1995; Kathren and Ehrhart, 1998) is conducting a study to obtain tissue at autopsy from workers exposed in facilities processing uranium. To date no long-term renal changes have been observed. Their publications provide an abundance of human tissue data.
Inhalation--Radiological Toxicity. Negative health effects resulting from the radiation effects of depleted or natural uranium have not been observed in humans. (See Appendix D for a discussion of single particle lung dosimetry.) There is evidence of lung cancer in miners from epidemiological studies, but this is related to exposure to a combination of airborne short-lived decay products of radon and other air toxicants, such as silica dust, diesel fumes, and cigarette smoke (NCRP, 1978; NIH, 1994; NRC, 1988). Uranium mill workers have not shown excess lung cancer or other disease despite their increased exposure to uranium and radon progeny. A population of workers was exposed to insoluble uranium dust (including UO2) at levels of 0.5-2.5 mg U/m3 with some exposed up to an estimated 10 mg U/m3 for about five years. None of these workers were exposed to other potential irritants. They did not exhibit respiratory disease (Eisenbud and Quigley, 1956).
Lung damage resulting from inhalation of uranium oxides is usually noncancerous alveolar epithelium damage of type II cells. The responses to chronic injury are hyperplasia, hypertrophy, and metaplasia (HHS, 1997b). In animal studies, lung and tracheobronchial lymph node fibrosis was reported in animals exposed by inhalation to 5.1 mg UO2/m3 (3.4 nCi/m3) for three years (Leach et al., 1970).
Animal studies have also examined pulmonary damage from exposure to uranium oxides. Exposure to 5 mg UO2/m3 for more than three years did not result in damage to the lungs, but minimal fibrosis, suggestive of radiation injury, was occasionally observed in the tracheobronchial lymph nodes of dogs and monkeys and lungs of monkeys (Leach et al., 1970, 1973). Alpha radiation doses were estimated to have been greater than 500 rads in the lungs and greater than 7,000 rads in tracheobronchial lymph nodes. Lung fibrosis is consistent with relatively short-term high exposure rate in animal experiments.
Stokinger (1953), on the other hand, found that exposure to concentrations up to 10 mg UO2/m3 for up to one year was tolerated by dogs. Likewise, Dygert (1949) observed no evidence of injury to the lung resulting from exposure to 17 mg U3O8/m3 for 26 days. Only very slight pulmonary change was observed in dogs and rats exposed to 19 mg UO3/m3 for four weeks.
Cancer rates in populations of highly exposed uranium industry workers have been examined. The rate of lung and skin cancer in these highly exposed populations was investigated in a follow-up study of mortality. Among 18,869 white males employed between 1943 and 1947 at a uranium conversion and enrichment plant in Oak Ridge, Tennessee, no excess cancers were observed through 1974 (Poldenak, 1981). This study, carried out at Oak Ridge National Laboratories, continues. Several other published epidemiological studies of uranium mills and metal processing plant workers have either found no excess cancer or documented that excess lung cancer was attributable to other known carcinogens (radon and its progeny and cigarette smoke) rather than uranium (Poldenak, 1981, 1982; Cragle, 1988; Reyes, 1984; Saccomanno, 1982; Hadjimichael, 1983; Carpenter, 1988). Although not statistically significant, a dose-response relationship between lung cancer and cumulative gamma radiation in a nuclear materials fabrication plant was noted by Checkoway (1998). (See Appendix E for a discussion of risks associated with radon exposure.)
Osteosarcoma (bone cancer) has been examined in the human studies. No association between osteosarcoma and uranium exposure was reported (Archer, 1973a; Cragle, 1988; Poldenak, 1981, 1982; Reyes, 1984; Saccomanno, 1976; Hadjimichael, 1983). Two studies link lymphatic malignancies to uranium exposure in uranium millers, but the authors suggest that 230Th (thorium-230), rather than the uranium, was the causative agent (Waxweiler et al., 1983; Archer, 1973b). Cragle (1988) observed an excess in leukemia deaths but was unable to determine if there was an association with radiation. A variety of cancers, including leukemia and carcinoma of the lungs and kidney, developed when rats were exposed to enriched uranium (92.8 percent 235U). However, because DU is orders of magnitude less radioactive than enriched uranium, these data are of little relevance to possible radiation-related health effects of DU exposure.
High exposure to radiation may also be nephrotoxic (Goodman, 1985). Evidence in the mouse and dog suggests that combined alpha irradiation from enriched uranium and metallotoxicity may produce a greater nephrotoxicity than doses of either separately (Wrenn et al., 1987). Likewise, some evidence suggests that enriched uranium may affect the immune system (Morris et al., 1992). However, there is no evidence that natural or depleted uranium has the capacity to induce this toxicity.
GI effects resulting from high levels of inhalation have been reported. In a case study, a male worker at a uranium-enrichment plant was accidentally exposed in a closed room to inhalation of a high concentration of UF4 (estimated radioactivity of 187 nCi/m3) for about five minutes. Six days after the accident, the patient reported dizziness, nausea, and loss of appetite. On the ninth day after the accident, the clinical findings were loss of appetite, abdominal pain, diarrhea, tenesmus, and pus and blood in the stool. By the thirtieth day after the accident and at follow-up seven years later, all clinical findings had returned to normal (Zhao and Zhao, 1990). The clinical findings were undoubtedly due to the chemical effects of UF4. In corresponding animal studies, exposure to enriched uranium damaged elements of the GI tract.
The issue of reproductive effects of inhaled uranium is discussed below (see "Reproductive Effects of DU" p. 63).
To summarize, cancer is the only radiation-associated disease that has been shown to be related to inhalation of radioactive particulates. For external gamma ray radiation, such as to atomic bomb survivors, there is a linear relationship with increasing dose yielding increased cancer risk. The best estimates are that a whole body effective dose of 1,000 mSv yields a cancer risk of about 4 percent. However, no evidence is documented in the literature of cancer or any other negative health effect related to the radiation received from exposure to natural uranium, whether inhaled or ingested, even at very high doses. The biological properties of uranium in the body and its absorption from the GI tract are reasonably well known from occupational exposures, studies of normal environmental intake, and animal studies.
The radiation dose can be calculated for any body organ given the amount in the organ. Based on the distribution in the body and the known body organ content, no health effects from radiation would be expected even for high occupational exposures. This results mainly because of the low radioactivity of natural uranium and the inability to get enough into the body to deliver a radiation dose that could be significant in causing cancer. The same would be true for DU.
Natural uranium is inhaled daily in very small amounts by all persons. Estimates made from measurements of air concentrations in New York City show that about 1 �g of uranium is inhaled each year by each person. The source of this uranium is mostly resuspended soil particles of very small diameter.
It is important to note that 99 percent of the mass or weight of natural uranium or DU is always associated with the 238U isotope and not the other isotopes of uranium (234U, 235U). Its low radioactivity per unit weight results from the much longer half-life of 238U compared with that for the other two isotopes. Thus, in order to inhale a significant quantity of radioactivity, the air concentration must have large enough mass to be visible.
Any atmosphere considered dusty contains at least a few milligrams of mass per cubic meter (mg/m3 equals 1,000 �g/m3), and the particles can usually be seen. Occupational uranium exposures of this kind have occurred, for example, when uranium metal was handled by a worker and exploded on contact with air. These extraordinary exposures resulted in inhalation of tens of milligrams of uranium. The uranium in these cases was rapidly excreted in urine, with more than 99 percent excreted in less than one week, and no subsequent health effects or cancer have ever been seen (see Figure 2.9).
A great deal can be learned about radiation dose and organ and tissue retention from the natural uranium content of the body. This uranium is obtained normally throughout life both from daily inhalation intake and through ingestion of food. These data show quantitatively the steady-state tissue concentrations from continuous intake from sources that cannot be avoided.
For example, the inhalation intake from breathing New York City air was measured as about 1 �g per year, and the uranium is known to be in an insoluble form (see Figure 2.4). Figure 2.4 shows the uranium lung tissue concentration in the lungs as a function of age. Over a 40 year period, the lung content increased from 0.4 �g by about 0.4 �g to a final value of 0.8 �g. The amount of uranium actually inhaled during 40 years, is 40 years x 1 �g per year or 40 �g. The increase of 0.4 �g in the lung suggests a retention of 1 percent, indicating the very efficient removal of inhaled uranium by the body.
The radiation dose factor (effective dose) for the lung from natural uranium is 0.001 mSv (0.1 mrem) per year per �g U per kg of lung. For New York City inhabitants, this translates to a radiation dose of 0.0005 mSv (0.05 mrem) per year and can be compared with an effective dose to the lung of 2 mSv (200 mrem) per year from other sources of inhaled natural radioactivity.
Thus, an unreasonable amount (2,000 �g) of DU would need to be inhaled to approach the existing dose from other natural exposures. An effective dose of less than 0.01 mSv (1 mrem) per year is considered a negligible exposure by the radiation guidance committees of NCRP and ICRP.
It is unlikely that any munitions explosion involving DU could have sustained air concentrations of DU in the mg/m3 range (1,000 �g/m3) for any length of time. Outside of struck vehicles dispersion of airborne material by normal wind speeds and ground deposition (fallout) dilute any clouds of material rapidly. (See OSAGWI, 1998, and CHPPM, 1998, for discussions of exposure).
Thus, based on the general literature, exposure to uranium of a large enough dose to be of radiological significance seems unlikely in the Gulf War; however, other groups such as the Office of the Special Assistant to the Secretary of Defense for Gulf War Illnesses (OSAGWI) (1998) and CHPPM (1998) are evaluating the level of exposure to DU in the Gulf War. Inhaled uranium is excreted efficiently, and long-term effects are unlikely because the radiation dose to organs is very small, i.e., much less than normal background radiation.
Even very small quantities of uranium, including background exposure, can be detected in a 24-hour urine analysis. As the body reaches a steady state (see the compartmental model in Figure 2.2) and as these steady-state values are known, if one knows normal background levels of excreted uranium, one can estimate industrial or battlefield exposure.[7] For this reason it is always important to obtain samples for both biological and environmental background levels so that quantitative estimates of exposure can be made without ambiguity. Urine samples can be used to predict total exposure and environmental soil samples show ground deposition, which can be used to determine the inventory of material dispersed.
Another major pathway for human exposure to uranium is through ingestion. The major portion of the body burden of uranium in the general public is derived from ingestion of food and drinking water. Depending on where one lives and on one's diet, exposure to natural uranium varies and continues over an entire lifetime.
In addition to uranium intake from food and water, some uranium ingestion may also follow hand-to-mouth contact in presence of uranium-containing dusts. As discussed in the inhalation section, uranium particles deposited in the upper airways are cleared from the lungs by mucociliary action, swallowed, and eventually reach the GI tract.
Uranium is not efficiently absorbed from the intestinal lumen, as is the case with other heavy metals (Spencer et al., 1990). For instance, fractional uptake of cadmium from the adult intestine is much lower (1 to 5 percent) than that from the alveoli (Friberg, 1985);[8] however, the immature gut is generally much more permeable to cadmium than the mature organ (Foulkes, 1993). The actual uptake of heavy metals from the intestine is determined by the interplay of many factors besides age. They include the chemical species of the metal present in the lumen; the composition of the intestinal contents and the presence of metal chelators and nondigestible metal-binding polymers, nutrition, and such physiological variables as pregnancy and lactation; and others (Foulkes, 1994).
The generally very low fractional absorption of ingested uranium in the mature human is consonant with the observation that fecal excretion approximately equals oral intake (Voegtlin and Hodge, 1949; Hursh and Spoor, 1973; Spencer et al., 1990). This finding also excludes the occurrence of significant hepatic or enteric excretion of the metal under normal conditions. More than 90 percent of uranium taken up by the GI tract to blood is rapidly excreted in urine (Wrenn et al., 1985). At steady state (i.e., continuous intake), the urine-to-diet ratio equals the fractional uptake of uranium.
There is some controversy concerning the relative uptake from water and food. The daily intake of uranium in food is estimated to be from 1 to 2 �g/day (Hamilton, 1972; Welford, 1967; Fisenne et al., 1987). In a few places, the intake of uranium from drinking water may exceed that from food (Cothern, 1983).
Uptake measurements from food and water separately were made in the only controlled dietary study in the metabolic research ward in the VA Hospital at Hines, Illinois (Spencer et al., 1990). Four patients, diagnosed for psychoneurosis, received a standard diet for weeks or months prior to uranium measurements. The uranium measurements on diet and excreta were made over a period of 24 to 54 days. In the Spencer et al. study (1990), when total diet was used, the uptake to blood of 234U and 238U were 1.8 and 1.5 percent, respectively. However, the water consumed by the patients had a relatively high uranium concentration of 10.4 and 7.3 mBq/L of 234U and 238U, respectively, stemming from natural sources in the region.
The four patients' water intake varied from 0.9 to 3.6 liters per day. The linear regression of urinary output versus uranium intake in water was very good, with 5 percent and 4 percent uptake for 234U and 238U, respectively. Water was not given with food in this study because water could have an effect on solubility of uranium in food. The uptake is therefore from the water itself. It appears from this one controlled study that uptake of uranium from water is greater than that estimated from the total diet. This finding generally holds for heavy metals.
Fractional GI uptake for uranium is known to vary inversely with the amount ingested. The fractional GI uptake (blood/diet) in historical human experiments ranged from 0.005 to 0.05 (Hursh et al., 1969). The ICRP (1979) has used 0.05 for water-soluble inorganic compounds of uranium (hexavalent uranium). The most recent ICRP value for GI uptake of unspecified compounds of uranium is 0.02 (ICRP, 1997). In a study of 234U and 238U administered in a single 3,000 mBq dose in water, the uptake as measured by urinary excretion ranged from 0 to 2.5 percent for 12 subjects (Wrenn et al., 1988).
Relatively few measurements are available for the uranium concentration in food products. Data for uranium in the diet are commonly estimated in four ways: the concentration in individual items is measured and intake estimated; a collection of typical individual food items are analyzed as a group in the "market basket" technique; typical meals are prepared and composited over several days and analyzed as a single sample; or fecal excretion is measured and analyzed (Singh, 1990). Each method has its merits.
Early estimates were made of uranium in the composite diet of six countries: France, West Germany, USSR, United Kingdom, Japan, and the United States and were summarized by Harley (1988). These data are shown in Table 2.3.
Table 2.3
Daily Dietary Intake of 238U in Various Countries
| Country | Region | 238U (mBq/day) |
| France | 12 | |
| West Germany | 30 | |
| USSR | Moscow | 45 |
| United Kingdom | 12 | |
| Japan | Sapporo | 18 |
| Kyoto | 18 | |
| Okayama | 11-60 | |
| United States | Chicago | 17 |
| New York, 1963 | 16 | |
| New York, 1978 | 15 | |
| San Francisco | 16 |
SOURCE: Harley, 1988.
Table 2.4
| Food Type | Consumption (kg/yr) | 238U (mBq/yr) | 234U (mBq/yr) | 235U (mBq/yr) |
| Annual | ||||
| Fresh vegetables | 48 | 1,142 | 1,128.0 | 58.0 |
| Canned vegetables | 22 | 92 | 99.0 | 2.6 |
| Root vegetables | 10 | 77 | 125.0 | 1.3 |
| Potatoes | 38 | 34 | 41.0 | 1.9 |
| Dry beans | 3 | 83 | 93.0 | 4.0 |
| Fresh fruit | 59 | 118 | 118.0 | 0.0 |
| Canned fruit | 11 | 11 | 29.0 | 0.2 |
| Fruit juice | 28 | 17 | 15.0 | 0.0 |
| Bakery products | 44 | 1,012 | 1,305.0 | 56.0 |
| Flour | 34 | 164 | 129.0 | 8.5 |
| Whole grain products | 11 | 187 | 277.0 | 11.0 |
| Macaroni | 3 | 11 | 11.0 | 0.3 |
| Rice | 3 | 9 | 7.5 | 0.5 |
| Meat | 79 | 184 | 145.0 | 1.3 |
| Poultry | 20 | 16 | 15.0 | 1.3 |
| Eggs | 15 | 28 | 15.0 | 0.5 |
| Fresh fish | 8 | 105 | 145.0 | 3.3 |
| Shellfish | 1 | 1,935 | 2,200.0 | 90.0 |
| Dairy products | 200 | 147 | 200.0 | 10.0 |
| Daily | ||||
| Dietary intake (mBq) | 14.700 | 16.800 | 0.6900 | |
| Water intake (mBq) | 1.220 | 1.460 | 0.0490 | |
| Inhalation intake (mBq) | 0.019 | 0.019 | 0.0007 | |
| Total intake (mBq) | 15.900 | 18.300 | 0.7400 |
SOURCE: Fisenne, 1987.
Items from 19 individual food categories and tap water in New York City were analyzed for isotopic uranium (234U, 235U, 238U) (Fisenne et al., 1987). Annual consumption of these 19 categories were estimated, and total uranium consumption was calculated for each isotope. The average daily intake in New York City was 18.3 � 0.5, 0.74 � 0.08, and 15.9 � 0.4 mBq for 234U, 235U, and 238U, respectively. The main contributors to the diet are shellfish, bakery products, and fresh vegetables. The average daily intake included inhalation intake, estimated from measurements in filtered air samples in New York City. The daily intake in the 19 categories and the water and inhalation intake are shown in Table 2.4.
The 238U in the diet in 31 cities in Japan was measured. Each sample consisted of the total of the daily whole meals collected from five adult males in one sampling place. The average daily intake in the 31 cities was 0.71 � 0.32 �g/day (8.5 � 3.8 mBq/day). The data are shown in Table 2.5.
Table 2.5
Daily Intake of 238U in 31 Locations in Japan During 1981a
| 238U �g/day | 238U �g/day | |||||
| Location | Summer | Winter | Location | Summer | Winter | |
| Sapporo | 1.06 | 2.94 | Osaka | 0.40 | 0.91 | |
| Aomori | 1.23 | 0.54 | Hyoto | 0.75 | 0.65 | |
| Miyagi | 0.55 | 0.93 | Wakayama | 0.56 | 0.45 | |
| Akita | 0.70 | 0.34 | Tottori | 0.45 | 0.37 | |
| Yamagata | 0.74 | 0.79 | Shimane | 1.01 | 0.54 | |
| Fukushima | 0.70 | 0.49 | Okayama | 0.95 | 0.69 | |
| Tokyo | 1.24 | 1.50 | Hiroshima | 1.38 | 0.32 | |
| Kanagawa | 0.89 | 1.11 | Yamaguchi | 0.40 | 0.42 | |
| Ibaraki | 0.87 | 0.84 | Ehime | 0.73 | 0.36 | |
| Nagano | 0.70 | 0.52 | Kochi | 0.32 | 0.24 | |
| Niigata | 1.21 | 0.47 | Fukuoka | 1.20 | 0.30 | |
| Isihawa | 0.76 | 0.90 | Saga | 0.89 | 0.45 | |
| Fukui | 0.89 | 0.60 | Nagasaki | 0.52 | 0.26 | |
| Shizuoka | 0.63 | 0.50 | Kagoshima | 0.32 | 0.32 | |
| Aichi | 1.28 | 0.87 | Okinawa | 0.91 | 0.87 | |
| Kyoto | 1.12 | 0.98 | ||||
SOURCE: BEIR IV, 1988.
aThe mean for the areas tested was 0.72 � 0.32.
A market basket study of uranium in the diet in Mito, Ibaraki prefecture, Japan, found an average daily intake of 15.5 � 0.8 mBq/day. The authors state that imported foods would increase this average by a factor of 1.6. The measurements were performed by ICP-MS and are considered normal levels. The committee on the Biological Effects of Ionizing Radiation reports that eating food or drinking water that has normal amounts of uranium will not likely cause cancer or other health problems in people (BEIR IV, 1988).
Ingestion--Chemical Toxicity. The chemical toxicity of ingested uranium is determined largely by the water solubility of the compound and therefore, ease of uptake from the GI tract. In general, compared with the industrial, the uranium oxides are considered to be less soluble or insoluble and, therefore, of very low toxicity (Tannenbaum, 1951; Maynard, 1949). Intravenous administration of uranyl salts in several animal species tested (for review see Bentley et al., 1985) produces signs of renal malfunction; the threshold dose is suggested to lie at 10 �g/kg body weight.
There are no studies that report human deaths from oral exposure to uranium oxides (HHS, 1997b). No mortality was observed in mice dosed at 0.02 to 20 mg UO2/day/mouse for a year (Tannenbaum, 1951). Likewise, no mortality and no evidence of toxicity appeared in mice dosed 100 mg U3O8/day/mouse for a year (Tannenbaum, 1951). Maynard observed no toxicity in rats fed a diet up to 20 percent uranium by weight in the form of UO2 or U3O8 or up to 0.5 percent uranium in the form of UO3. Mortality can be induced in animals at very high oral intake levels. One hundred percent mortality was observed in a 30-day rat study with a diet consisting of 2 percent uranium in the form of UO3 (Maynard et al., 1953).
No human studies were located related to respiratory, cardiovascular, hematological, musculoskeletal, hepatic, renal endocrine, dermal, ocular, body weight, or other systemic effects in human exposure to uranium compounds through the oral route (HHS, 1997b).
Respiratory effects have not been observed in animals following ingestion of uranium oxides. In animals fed diets of 20 percent uranium in the form of UO2 for 30 days, no adverse effects on the respiratory system were found (Maynard and Hodge, 1942; Maynard et al., 1953). Likewise, no adverse cardiovascular effects were observed in animal studies following oral exposure (Maynard and Hodge, 1942; Maynard et al., 1953).
The adverse effects on the GI tract were observed following a case study of a human volunteer (Butterworth, 1955). Following a single dose of uranyl nitrate (14.3 mg/kg), a compound considered far more toxic than an oxide, acute nausea, vomiting, and diarrhea occurred within a few hours. Within 24 hours, no clinical effects remained (Butterworth, 1955).
No studies were located that noted an association between uranium oxides and hematological parameters (HHS, 1997b). Immune system effects have not been attributed to uranium oxide exposure (Maynard et al., 1953). Although hepatic effects have been observed in animals dosed with very high levels of insoluble uranium, dogs dosed with up to 10 g UO2/kg/day for one year exhibited no hepatic effects (Maynard and Hodge, 1949).
No animal studies were located that examined effects of ingested uranium oxides on the endocrine system. No harmful effects on body weight were seen in intermediate-duration oral studies of dogs given up to 10 g UO2/kg/day for one year (Maynard and Hodge, 1949; Maynard, 1953).
No epidemiological studies were located that examined neurological effects following uranium ingestion. And no animal studies examined the effect of uranium oxides on the neurological system (HHS, 1997b).
Reproductive, developmental, and genotoxic effects have also been investigated. No studies were located that reported reproductive effects in humans following oral exposure to uranium for any duration (HHS, 1997b).
Gene mutation and chromosomal aberrations following alpha radiation exposure emitted by uranium decay products might be a concern. However, no studies were located that reported genotoxic effects in humans or animals following oral exposure to uranium for any duration.
The possibility of teratological actions of ingested uranium was looked for in mice. Developmental effects, such as reduced fetal body weight and length, and external malformations have been observed with nonoxide uranium compounds (Domingo et al., 1989); a no-effect-level of below 5 mg uranyl acetate dihydrate per kg per day during pregnancy was suggested. No such studies have examined developmental effects associated with uranium oxide ingestion. Also, no studies were located that reported reproductive effects in humans following oral exposure to uranium for any duration.
While possible toxic effects of uranium on other organ systems have not been rigorously excluded, extensive work points to the kidney as the major target organ. The recent work of Zamora et al. (1998) is therefore of special interest. Zamora et al. described analyses on renal function after a lifetime of exposure to natural uranium of 20 control subjects consuming drinking water containing less than 1 �g U/L and 30 subjects exposed to higher uranium levels (range 2-781 �g/L). The higher-exposure group took in a total of 3-570 �g/day; intake by the control group amounted to 0.3-20 �g/day. The exposed group excreted significantly more glucose in the urine than did the control group. Excretion of alkaline phosphatase (a marker of cytotoxicity) and of �2-microglobulin (a marker of proximal tubular function) were positively correlated with uranium intake. The only valid measure of glomerular malfunction tested (proteinuria) remained unchanged. No clearances were reported, and a constant urinary excretion of creatinine can, of course, throw no light on the glomerular filtration rate. In the absence of information on the reversibility of the observed changes in tubular function, their clinical significance must remain in question. This is especially true because most of the functions measured remained within the reference (normal) limits.
A more general question then arises: when should a subclinical effect be labeled adverse? There is no consensus on this problem. Thus, would the decreased body weight looked for in the work of Maynard et al. (1953), as quoted above, have represented a harmful effect? The possibility of acquired partial tolerance to several heavy metals, including uranium, was demonstrated in animals upon preexposure to low levels. As already discussed above under the heading of tolerance to inhaled uranium, acquired resistance to, for instance, cadmium can be explained in terms of the induction of the metal-binding protein metallothionein; no such role of metallothionein has been demonstrated in uranium exposure.
No nephrotoxic effects were observed in dogs fed 1 g UO2/kg/day for one year. Moderate to severe histological effects were observed in dogs fed 10 g UO2/kg/day for one year (Maynard and Hodge, 1949). Regeneration of damaged tubular epithelium has been observed in animal studies after discontinuation of exposure (Bentley et al., 1985; Dygert, 1949; Maynard and Hodge, 1949; Rothstein, 1949; Stokinger et al., 1953). The regenerated epithelial cells appear different from normal.
In conclusion, there is only limited evidence to suggest that even chronic exposure to natural uranium in food or drinking water, except at very high concentrations, is associated with increased morbidity in humans or animals. This conclusion makes it unlikely that DU would have any such effects.
Ingestion--Radiological Toxicity. No human or animal studies associate adverse health effects with the radioactivity of ingested DU. No evidence has been found to associate human exposure to ingested uranium compounds and carcinogenesis. Likewise, no oral animal studies reported the evidence of cancer induction (Maynard and Hodge, 1949; Maynard et al., 1953; Tannenbaum and Silverstone, 1951, Kathren and Moore, 1986).[9] Note that much of this evidence and discussion was presented earlier in the report as considerations related to the radiological effects of inhaled and ingested uranium are similar.
The major pathway for entry of natural uranium into the body is through water and food. In a weapons explosion involving DU, ingestion of material could certainly result by directly swallowing airborne material or ingesting soil contaminated with DU. Also, most inhaled uranium is cleared by the lung and swallowed, mimicking ingestion.
The radiation dose to the kidney in New York City residents, using the measured kidney concentration of 0.43 �g/kg (0.00043 �g/g), is 0.0004 mSv (.040 mrem) effective dose per year. Ingestion of DU in a munitions explosion or fire would likely be short-term with small fractional uptake and subsequent rapid excretion of absorbed uranium reaching the blood. The radiation dose to the kidney from the de facto standard of 3,000 �g/kg kidney would deliver an effective dose of 4 mSv (400 mrem) per year. This is less than the small group limit for infrequent exposure of 5 mSv (500 mrem) per year and the occupational limit of 10 mSv per year (See "Regulatory Standards," p. 8). Continuous uptake is unlikely, except in the case of embedded uranium in a wound. In a wound, the solubilized uranium presents a continuous daily input to blood and is equivalent to continuous intake from food or water. This is described later.
Today, quantitative estimates of exposure to detect even minute concentrations of uranium in bioassay (urine or tissue) samples can be made with high sensitivity and accuracy. Even in the case of single short-term exposure, it is sometimes possible to measure excretion concentrations long after exposure if the exposure is sufficiently large and base-line levels are known. Bioassay samples can sometimes be used in this way to estimate retrospectively the total exposure and radiation dose.
Urinary Excretion: The best body fluid in which to measure uranium is urine because soluble uranium, once absorbed, is rapidly eliminated from the body through the kidney (Lauwerys, 1993). Excretion basically occurs as a two-phase process with 70-86 percent being excreted in the first 24 hours, and the remainder excreted over the period of many months (Berlin, 1986).
As an example, a worker was exposed to massive amounts of uranium fumes by inhalation and ingestion following the sudden accidental explosion of powdered uranium metal. The rapid excretion of uranium by this worker is shown in Figure 2.9 (Eisenbud and Quigley, 1956). Figure 2.9 shows that in the first 10 days, levels dropped extremely rapidly from more than 20,000 to about 10 micrograms of uranium per liter of urine.
In unexposed individuals, almost all daily intake of uranium comes from drinking water and ingestion of such foods as vegetables, grains, and salt (Berlin, 1986; Fisenne, 1987). In the absence of an acute environmental exposure, inhalation of uranium accounts for less than 2 percent of the contribution from diet (Dang, 1992). Absorption from skin is also possible if uranium sources are water soluble. Water-insoluble compounds (e.g., metal armor) have not been detected (Berlin, 1986). From water and diet, the GI absorption factor itself (the percentage of ingested uranium actually absorbed) is about 2 to 5 percent. Studies of unexposed individuals have shown that urinary concentrations average 12.8 ng/L (Dang, 1992) with a daily excretion of 30.9 � 19.6 ng/24 hours (Medley, 1994).
Following acute inhalation, little uranium reaches the lower lung, as was previously discussed. This is because particle size is such that most is captured by the respiratory tract (and ultimately swallowed). Estimates suggest that from 1 to 5 percent of uranium-containing dust will penetrate to the pulmonary parenchyma and ultimately be absorbed (Berlin, 1986).
Studies suggest that considerable amounts of uranium must be present in the circulation to cause significant renal damage. Studies in dogs suggest that 10 mg/kg intravenously produced acute renal failure. In nonlethal doses, renal injury presents with evidence of tubular damage (cellular and noncellular casts on urinalysis). After several days, the renal tubular epithelium begins to regenerate and is generally complete by two to three weeks (Berlin, 1986). While at first the regenerated tubular cells appear structurally different, they ultimately regain their normal appearance. However, function is restored within weeks of the start of epithelial regeneration.
Berlin and Rudell (1996) report that uranium concentrations in urine of up to 2.84 mg/L have been observed without clinical evidence of renal damage. This concentration exceeds 220,000 times more than has been reported in unexposed subjects (Dang, 1992). From the standpoint of occupational exposure, the United States Energy Research and Development Administration (1975) set a maximum safe limit at 250 �g/L, a concentration about 20,000 times the average background.
With the exception of individuals with embedded DU fragments, measuring urinary uranium in Gulf War veterans at this time would not be expected to provide useful information. Previously exposed individuals would now be expected to have urinary levels within the range of unexposed individuals, perhaps up to about 300 ng/L (Dang, 1992). In the absence of acute renal failure in the Persian Gulf, which would have been obvious shortly after exposure, medical and experimental evidence does not suggest that veterans are suffering from long-term renal uranium toxicity. Routine monitoring or evaluation of veterans for evidence of renal damage (e.g., serum creatinine, blood urea nitrogen, microalbuminurea) is not warranted.
If in the future the possibility of acute exposure to uranium is considered, urine should be collected in the first day following the event. Efforts should be made to collect a 24-hour specimen for analysis because studies have documented considerable variation in urinary uranium concentrations among those with nontoxic occupational exposures (Medley, 1994).
In summary, the databases developed for tissue concentrations of uranium acquired from normal dietary intake are of major importance. They permit a quantitative value for uranium organ content per unit intake from the natural uranium content of diet. Controlled studies of dietary intake of natural uranium showed that uranium in food is poorly absorbed by the gut compared with uranium in water. Uranium in water is in a more available form. Under natural conditions, i.e., intake of a few �g per day, the fractional uranium uptake from water to blood is about 5 percent (Spencer et al., 1990). With increasing mass of uranium, the gut is less able to absorb uranium and the fractional uptake to blood is less.
The dietary intake of uranium from food and water in New York City is about 1 �g per day. The steady-state uranium organ burdens associated with this intake are shown in Figure 2.4. This is a powerful relationship as the organ content for other continuous exposure situations can be calculated readily. For example, the kidney concentration in New York City residents is 0.4 �g/kg and, assuming this arises from water, means that for ingestion of 1 �g per day from water the kidney concentration would be 4 �g/kg. The de facto standard (MPC) for uranium in the kidney is 3,000 �g/kg of kidney. Again, it is unlikely that an ingestion exposure to DU would be as high as 1,000 �g per day, or enough to attain the guideline concentration for kidney. For short-term or single exposures, the radiation dose would be less than for the same amount given continuously. The dosimetry for specific individuals or case events should be derived for the known conditions of ingestion exposure.
Exposure to ingested, inhaled, or embedded uranium can be diagnosed by looking at the level of uranium in the urine through urinalysis. Urinary excretion is a positive, rapid, and unambiguous identification of exposure to ingested or inhaled uranium. Once in the body, any uranium compound will be soluble to some degree and cleared to the blood. When in the blood, any uranium is rapidly excreted in urine.
Embedded Fragments and Wound Contamination
Embedded DU fragments and wound contamination with DU dust is another pathway for exposure, seen almost exclusively in the military environment. In combat use, DU metal fragments from a penetrator or a vehicle's hull armor can scatter inside the vehicle, killing and injuring personnel, destroying equipment, and causing secondary explosions and fires. As the use of DU munitions is relatively recent, there is little published literature on exposure to embedded fragments.
As a result of "friendly fire" incidents during the Persian Gulf War, DoD has reported that DU munitions struck a number of Bradley Fighting Vehicles and Abrams Tanks[10] (OSAGWI, 1998). The friendly fire incidents killed 13 soldiers and wounded many more (GAO, 1993). The total number of soldiers wounded by DU is not known; however, the Office of the Army Surgeon General identified 22 soldiers whose medical records indicate they have embedded fragments that might be DU. Another 13 soldiers were wounded and hospitalized but were not specifically identified as having fragment wounds. The remaining crew members of the DU-struck vehicles were either not wounded during the incident or had minor wounds that were treated in the field (Daxon, 1993; Kearsley, 1993).
Following the Gulf War, the Office of the Army Surgeon General requested the Armed Forces Radiobiology Research Institute (AFRRI) to "assess the health risks associated with implanted DU fragments in the body to provide medical guidance for current and future patients with these fragments, and provide recommendations for future research." (Daxon, 1993.) Following a review of the medical literature, AFRRI concluded that this situation is radiologically and toxicologically unique. Until recently, there was no human or animal literature evaluating the health effects of embedded DU fragments. As such, the federal government has since funded research at AFRRI and the Inhalation Toxicology Research Institute (ITRI), University of New Mexico, to understand the behavior, physiology, histology, and biokinetics, as well as the carcinogenic potential of embedded DU metal. The research continues.
In addition, since the exposure has not been studied, the AFRRI recommended long-term follow-up for the affected soldiers. However, no compelling evidence to change standard medical criteria for fragment removal was found (Daxon, 1993). In response to the AFRRI recommendation, physicians and scientists from DoD and the VA drafted the protocol to be used in the follow-up effort. The protocol, reviewed and revised by a panel of experts, was submitted to the Army on December 7, 1992, and implemented in late 1993 as the Depleted Uranium Follow-Up Program at the Baltimore VA Medical Center. Since late 1996, the program has been under the clinical leadership of Dr. Melissa McDiarmid.[11]
The DU Follow-Up Program at the Baltimore VA Medical Center provides ongoing clinical surveillance to Gulf War veterans with known or suspected embedded DU fragments, DU-contaminated wounds, or significant amounts of inhaled DU aerosols (Daxon, 1993).
"The DU Follow-Up Program at the Baltimore Veterans Administration Medical Center began a medical surveillance program to assess the health of approximately 35 soldiers" (exact number was 33 (Kane et al., 1998)) in 1993 who had been in vehicles "struck by DU munitions. . . . Early phases of the program focused on the exposure assessment of these troops by measuring the early biologic effects of both the radiologic and presumed chemical toxicity of DU." (McDiarmid et al., 1998a.) The original research protocols included very thorough questionnaires and a series of medical and laboratory tests. Among other issues, questionnaires were designed to gather medical, social, family, reproductive, and occupational exposure histories. Tests were conducted to study renal function including urinalysis, creatinine, and proximal tubular function tests (�-2 microglobulin and retinol-binding protein). Other laboratory studies included blood chemistries, urinary uranium, and neuroendocrine measures. Among other tests and studies, patients also received detailed physical examinations, neuropsychological tests, and radiology tests (McDiarmid, 1998b).
The scope of the surveillance of the original patients was expanded in 1997, and this "more recent follow-up has attempted to identify the most sensitive and relevant biologic measures of uranium and have included uranium determinations in spot and 24-hour urines, seminal fluid and whole body radiation counting in both the DU exposure cohort and non-DU exposed Gulf War veteran controls. Early markers of proximal tubular effect and reproductive hormone values were also assessed." (McDiarmid et al., 1998a).
Currently, 33 participants are in the program. All were evaluated at the Baltimore VA Medical Center in 1993 and 1994, and 29 were reevaluated in 1997. Of those evaluated, about half have been identified radiographically as having retained metal fragments. In 1997, the majority of individuals identified radiographically as having retained DU fragments had elevated 24-hour urinary uranium levels (McDiarmid, 1998b). This suggests that DU is being oxidized in body fluids. Thus, these metal fragments are not entirely inert. Most individuals who did not have embedded fragments did not have elevated urinary uranium levels (McDiarmid, 1998b).
Twenty-six DU exposed participants in the DU Follow-Up Program at the Baltimore VA and 19 unexposed persons underwent whole body radiation counting (WBRC) (McPhaul et al., 1998). A summary statistic based on the WBRC result was derived for each participant, and this correlated highly with the 24-hour urinary uranium result (McDiarmid, 1998b).
Although these individuals have an array of health problems, many of which are related to their combat injuries, to date no manifestations of kidney disease attributable to the chemical toxicity of DU have been found; neither do these individuals appear to have manifestations attributable to radiation effects (McDiarmid et al., 1998a; McDiarmid, 1998b), but several perturbations in biochemical and neuropsychological testing have been correlated with elevated urinary uranium, the clinical significance of which is unclear (McDiarmid, 1998b). These patients continue to be followed to be sure that this remains the case.
Laboratory tests also found DU in semen in samples from some but not all veterans exposed to DU. This is not altogether surprising as DU disperses throughout the body. It is not clear what effect if any this might have on reproduction of a couple, where one partner has an embedded DU fragment. To date, all births to couples in the DU Follow-Up Program have been normal (McDiarmid, 1998b). There have also been no studies to determine the level, if any, of uranium from dietary intake appearing in semen in individuals living in areas with high levels of natural uranium.
Participants in the DU Follow-Up Program at the Baltimore VA Medical Center also participated in a set of standard tests to measure neurocognitive function. "As a group these individuals performed normally on standard tests of attention, memory, and problem solving. However, there was a statistical relationship between elevated urine uranium and lowered efficiency and accuracy" (Kane et al., 1998) measured in a follow-up computerized test. Researchers have urged caution in looking at these results as "an analysis of individual cases suggested that the performance of a few patients might have been responsible for this relationship on this relatively small sample of patients with elevated uranium levels. There was no evidence of worsening performance over time." (Kane et al., 1998.)
A series of important studies assessing the effects of embedded uranium in rats is presently being conducted by the Armed Forces Radiobiology Research Institute (AFRRI).[12] AFRRI is looking into the redistribution and toxicity of embedded DU fragments; in a separate study, it is studying the effect on DU on reproduction and fetal development (McClain, 1998). Male and female Sprague-Dawley rats weighing 250-300 g had four to 32 DU or tantalum control pellets (1 mm diameter by 2 mm in length) implanted in their legs (Benson and Pellmar, 1998b). Comparisons of exposure levels between rats and humans are difficult, but researchers indicate that the rats with the smallest number of DU pellets had a higher urinary uranium level than Gulf War veterans with embedded fragments, who were among the most highly exposed Gulf War veterans. A number of studies "to assess kidney function, uranium distribution, reproductive capability, behavior, and electrophysiology" (Benson and Pellmar, 1998b) were conducted.
At a recent conference and in a forthcoming paper (Benson and Pellmar, 1998a,b; Pellmar et al., forthcoming), researchers reported on preliminary results of their studies. "Uranium levels were high and dose-dependent in the kidney, urine, and bone [in DU implanted rats]. Despite high uranium levels in the kidney, no renal toxicity was evident." (Benson and Pellmar 1998b.) Twenty-four-hour urinary uranium levels in rats 12 months after implantation with 0, 4, 10, or 20 DU pellets were 0, 2, 4, and 11 �g, respectively. Unexpectedly, uranium was also found in hippocampus of the brain of DU implanted rats, but no neurotoxicity was evident (Benson and Pellmar, 1998a). Benson and Pellmar (1998a) concluded that "these data suggest that renal toxicity may be less of a hazard than anticipated, but that cognitive deficits need to be considered."
AFRRI is also examining the effects of implanted DU pellets on reproduction in female rats, measuring various maternal and litter parameters, including pup weight and litter size (see "Reproductive Effects of DU," p. 63). Results from mother rats with high levels of uranium indicate the pups are normal. Preliminary results suggest there may be a decrease in litter size in female rats impregnated six months after implantation with the largest number of DU pellets used (32) (McClain, 1998).
Elsewhere, it has also been reported that 27 members of the 144th Service and Supply Company, Army National Guard, potentially exposed themselves when they worked on DU-contaminated vehicles for three weeks without protective gear (AEPI, 1995). The CHPPM was assigned by Office of the Army Surgeon General to obtain and perform radio-chemical analyses on voluntary urine samples from personnel of the 144th. Among the members of the 144th Service and Supply Company, Army National Guard, tested at the CHPPM in 1993, no measurable increase in uranium excretion was found (OSAGWI, 1998).
From a radiological perspective, particles embedded in skin can irradiate the cells in the basal layer, the target cells for skin cancer. (Note: this refers to fragments embedded in the skin and not to DU radiological exposure from outside the body). For example, if a 100 �m fragment of DU was embedded near the basement membrane, it could irradiate a target cell, causing genetic damage that might result in skin cancer. The decay rate (i.e., alpha particle emission rate) of DU is very low for micrometer-size particles. However, the decay rate of a large 100 �m DU particle at 0.15 Bq is sufficient to kill all cells within the alpha particle range. In the case of an embedded fragment, damage to tissue next to the fragment would be caused primarily by alpha radiation while in the case of radiation external to the body, gamma and beta rays are the only rays that can penetrate the body and do damage.
The primary external health hazard from DU is beta and gamma radiation generated by the radioactive decay of uranium decay products. The vast majority of DU radiation (more than 95 percent) is alpha radiation, which cannot penetrate paper or skin and has been shown not to cause adverse external health effects. Gamma and beta radiation from decay products can penetrate the skin and in sufficient amounts could pose a health risk. Intact munitions and armor have the potential for amassing sufficient DU to generate enough beta and gamma radiation to exceed occupational levels. However, all DU weapon systems are shielded to control gamma and beta radiation emitted from DU. As such, they present very little external exposure risk for personnel working with intact munitions and armor (AEPI, 1995; Danesi, 1990). Chemical toxicity does not pose a threat unless the metal is internalized.
The potential radiological health effects from external DU exposure, including bare penetrator, armor, and dust, are small (AEPI, 1994). The dose of radiation received depends on several factors, including the amount present, the type and configuration of the DU, the distance from the DU source, and the time exposed.
The obvious target for external radiation exposure from DU deposited on the body is the skin. The alpha decay of a single particle is very low, with the additional factor of an increased distance from the skin surface to the target cell. The skin surface is composed of a layer of dead cells collectively called the epidermis. New cells are generated from basal cells in the stratum basale, a layer close to the basement membrane. Overlying the basal cells in the stratum basale are three other layers of cells: the stratum granulosum, the stratum lucidum, and the stratum corneum. The cells in the layers above the basal layer are terminally differentiated and therefore not susceptible to damage that could produce a cancerous growth.
The International Commission on Radiological Protection (1974) measured epidermal thickness in males aged 26 to 30 years. The average thickness is about 50 �m, and the thinnest epidermis is 34 �m on the front of the forearm. The range of the 238U alpha particle is 28 �m in tissue. Thus, most of the target cells are outside the range of particles deposited on the surface. The beta and gamma radiation from the decay products can irradiate cells in the basal layer.
Since alpha particles emitted by uranium will not penetrate the dead outer layer of the skin, the effects of acute dermal exposure to ionizing radiation, including erythema (redness of the skin) and epilation (loss of hair), will not be observed (Upton, 1992). Studies of uranium miners, millers, and processors have not reported these effects.
Likewise, only the putative stem cells (basal cells) that are cycling and that regenerate the cells in the upper layers are targets for carcinogenesis. Once cells are terminally differentiated, skin cancer cannot be induced. Out of 11 underground uranium mining cohorts studied, skin cancer was observed only in Czechoslovak and Chinese miners (Sevcova et al., 1983). In those studies that observed skin cancer, airborne arsenic, a known carcinogen, was also present in the mines and is considered to be the relevant substance. In addition, workers near nuclear reactors are subjected to skin deposition of small particles of highly radioactive activation products (denoted "fleas"). These particles attain decay rates of beta radioactivity of many thousands of disintegrations per minute. However, no skin cancer has been observed from this source. Likewise, no animal studies examining dermal exposure to uranium observed cancer after any duration of exposure.
The Army monitors soldiers and support workers according to NRC occupational exposure standards (10 CFR 20.1201) for beta and gamma radiation. The current occupational exposure radiation dose limit (beta and gamma) for skin is 50,000 mrem/year (500 mSv/year). Holding a DU penetrator without shielding (spent DU penetrator) would deliver a skin dose of approximately 200 mrem/hour (AEPI, 1995). The NRC occupational exposure standard could only be exceeded if a piece of DU were carried for more than 250 hours (AEPI, 1995).
No dermal effects and no body weight changes were seen following a single application of 1 to 2 g UO2 (mixed in lanolin) per rabbit to the shaved skin of rabbits (Orcutt, 1949). Using similar doses, no effects were observed for U3O8. However, mortality was observed following a single application of 1 to 2 g UO3 mixed in lanolin per rabbit to the shaved skin of rabbits (Orcutt, 1949). Sixty-seven percent mortality of New Zealand rabbits was seen with a single application of either 344 mg UCl5 per kg tissue or 666 mg UO3 per kg tissue (Orcutt, 1949). Orcutt also suggested that the order of species susceptibility to acute uranium toxicity was--from the most susceptible to least susceptible--rabbit, rat, guinea pig, pig, and mouse (Orcutt, 1949). DeRey et al. (1983) found that 100 percent, 60 percent, and 100 percent mortality rates were induced in male rats dermally exposed to 237 mg uranyl nitrate hexahydrate, 1,965 mg uranyl acetate, or 1,928 mg ammonium diuranate per kg of tissue, respectively. Chemically induced renal failure was responsible for all deaths (HHS, 1998). There are no recommended maximum permissible concentrations for exposure of human skin to uranium. However, the levels that induced mortality from dermal exposure in animals are extremely high. No human has ever died from dermal exposure to natural uranium (HHS, 1998).
No studies examined neurological effects from uranium oxide compounds; however, no neurological signs were observed in animals exposed to uranyl nitrate hexahydrate (Orcutt, 1949). No studies were located regarding the neurological effects for humans exposed to external uranium. Also, studies located for humans and animals do not describe any other effects, including reproductive, developmental, or genotoxic effects, following dermal exposure to uranium for any duration.
Radiation measurements inside a M1A1AHA tank have been taken. The decay products of 238U, 234Th (t1/2, 24.1 days) and 234Pa (t1/2, 1.17 min) build into equilibrium rapidly. The 234Pa emits penetrating gamma rays, and the potential for external whole body dose is present. The M1A1AHA tank contains DU armor and may contain DU munitions. The external gamma ray dose rate is stated to be 0.01-0.02 mrem/hr to the commander, loader, and gunner, while the driver's head can receive 0.13 mrem/hr with DU ammunition overhead (AEPI, 1995). These dose rates are assumed to be in excess of normal background radiation, which averages 0.01 mrem/hr inside a tank. The measurements of external gamma ray radiation are summarized in CHPPM, 1998, from measurements performed in the 1980s by Parkhurst et al. (see CHPPM, 1998; Parkhurst, 1991). These are shown in Appendix G and are below occupational exposure norms.
Low-level whole body radiation is generally defined as less than 0.01 Sv (10 rem). No effects have ever been observed in populations of humans or animals exposed to low-level radiation. Researchers are trying to determine whether low-level radiation increases risks of cancer or whether a threshold level exists. This is an important research need.
This section discusses the possible health effects associated with DU based on the research data previously presented. This discussion may be helpful to the clinician when evaluating a patient with possible exposure to DU. The clinical conditions discussed in this section are common. It is important, therefore, not to attribute conditions uniformly to Gulf War service when a veteran presents to his or her physician with findings generally observed in an otherwise matched civilian or unexposed military cohort. For this reason, statistics on common clinical conditions that have at times been theorized to be associated with DU are presented in the following discussion.
DU is a low-level radioactive heavy metal. As such, exposure to it in large enough quantities and in certain circumstances has the potential to cause adverse health effects by either chemical or radiological mechanisms. There is very little information on the health effects of DU; however, natural and enriched forms of uranium have been studied for decades and are well understood. Because DU is chemically identical to natural and enriched uranium, the toxicological health effects observed from those studied forms can be directly applied to DU. Because DU is 40 percent less radioactive than natural uranium and orders of magnitude less than enriched uranium, radiological health effects from DU are much less than from the more radioactive and better-studied forms of uranium.
Many human epidemiology and animal studies have been reviewed. Epidemiology is the tool used to evaluate the determinants and distribution of disease and injury in human populations. Epidemiology evaluates but cannot prove which factors or agents may cause or contribute to good or bad health effects. It is from epidemiological studies that we understand some very important health information--that tobacco smoking causes lung cancer and a low-fat diet reduces the risk of heart attacks, for example. Therefore, when epidemiological studies exist, public health professionals rely primarily on their results to evaluate potential health effects.
In contrast, animal studies examine how chemicals are absorbed, distributed, and excreted. Although it is usually difficult to extrapolate data from animal studies and apply the results directly to humans, for obvious ethical considerations humans cannot be subjected to laboratory tests where no potential benefit can accrue. Therefore, medical science and our regulatory apparatus routinely use and refer to animal studies for indications of possible health outcomes for humans, such as cancer and birth defects.
As mentioned, uranium is well understood, and we are all exposed to it in varying amounts every day. Fortunately, there are several large epidemiological studies involving uranium for us to examine and interpret. The studied populations were exposed occupationally to elevated concentrations of natural and enriched uranium over very long periods, including whole working lifetimes. In addition, there are case studies of acutely exposed individuals from occupational accidents. These exposures are far greater than known DU exposures during the Gulf War.
No human epidemiological studies are published that document mortality or detrimental respiratory, hematological, musculoskeletal, hepatic, renal, endocrine, dermal, ocular effects, or any other systemic health effects from uranium oxides (HHS, 1997b). The authors attributed many of the effects observed in populations exposed to uranium compounds to other causative agents.
Veterans of the Gulf War without embedded DU fragments tested in the last few years (years after the cessation of the Gulf War) have shown no increased urinary uranium concentrations. In contrast, individuals with evidence of retained fragments have shown increased excretion of uranium.
Diagnosis
Once exposure is suspected or known, the first step in the clinical process is diagnosis. The process and tests laid out below are fairly standard, but although DoD and the VA have agreed on appropriate steps of a DU evaluation, no clinical practice guideline for the treatment of exposure to DU exists.[13] Furthermore, the diagnostic process is properly determined by a physician in consultation with the patient. Almost all patients differ and must be treated accordingly.
If, after a detailed patient history, the clinical decision is made to evaluate a possible recent exposure to DU, a 24-hour urinary bioassay is usually the best method of determining whether exposure has taken place and at what level. Urinary excretion clears almost the entire uranium content of blood each day (80 to 95 percent). The analytical measurement is performed easily, and therefore, the majority of patients receive a 24-hour urinary uranium test following a recent exposure. Obtaining blood samples for uranium, in contrast, is much less valuable because of the rapid clearance of uranium in the urine; furthermore, the analytical technique is complicated by the blood matrix.
A 24-hour urine sample is preferred to a random spot urine specimen because the fraction of the daily amount excreted in a spot sample varies widely. Excretion of uranium does not progress at a constant rate throughout the day, so reliance on a spot sample is likely to yield misleading results. Although it is being debated in the scientific literature, some people believe that urinary uranium normalized for creatinine should be used.
Criteria have been developed to monitor occupational exposure to uranium, and urinary uranium assays have been used to evaluate ongoing exposure. The occupational criteria for determining whether the guideline of 3 �g/g kidney had been exceeded was 30 �g/L of urine. This is a fairly rapid assessment that can be performed to determine whether workers should be removed from an operation.
Results from work by McDiarmid (1998a and 1998b) with veterans and by AFRRI (Benson and Pellmar, 1998a) with rats indicate that urinary uranium concentrations in 24-hour urine collections are usually elevated when embedded fragments are present. In the case of embedded fragments, sometimes additional information can be gleaned through the use of scans. In some cases, whole body scans may be appropriate.
Whole body counting (sometimes called in vivo gamma spectrometry), however, is not a common diagnostic tool. Only a few institutions in the United States and abroad possess whole body counters. The most-sensitive instruments require massive shielding to lower the normal radioactive background.
The detection limit for measurement for natural uranium in a whole body counter is generally about 70-110 Bq (2-3 nCi) when the uranium body burden is distributed. This is equivalent to a body burden of approximately 10 milligrams. If a fragment is identified, localized counting at the body site is a more efficient method for the measurement, and somewhat less activity can be detected. The ability to detect DU depends on the detector used, the particle size, and the inherent shielding of the facility. Facility shielding is critical because DU radiation is so low that the background radiation would need to be exceedingly low to detect a difference.
While the testing described above is not usually medically indicated for Gulf War veterans who do not have embedded fragments, each patient must be individually evaluated by his or her physician, and the specific tests ordered will depend on symptoms and the findings that result from that evaluation.
Although uranium is radioactive, inhalation of uranium has not been demonstrated to pose a risk of cancer. However, many heavy metals are not radioactive and can cause cancer in humans. DU is an extremely weak radioactive material that has never been implicated in lung cancer in human populations. Individuals who inhaled DU that remains in the lungs (insoluble fraction) from exposures during Persian Gulf service should not be concerned that they harbor a serious threat to life. The literature suggests that there may be an increased risk of cancer among individuals exposed to enriched uranium, but that compound is orders of magnitude more radioactive than DU. Even exposure to natural uranium, with a radioactivity more than DU, is not considered to be a health threat. The lung cancer reported in uranium miners has been scientifically attributed to exposure to another airborne substance, namely radon (NCRP, 1984; NIH, 1994; NRC, 1998).
As a point of reference, lung cancer is the most common cancer in the United States today. The American Cancer Society estimates that there will be 171,500 new cases of lung cancer in 1998, accounting for 14 percent of cancer diagnoses. The incidence rate (annual) is declining in men, from a high of 87 per 100,000 in 1984 to 74 in 1994. Recently, the rate of increase among women has started to slow. In 1994, the incidence rate in women was 42 per 100,000.
Signs and symptoms of lung cancer include persistent cough, sputum streaked with blood, chest pain, and recurring pneumonia or bronchitis. Regarding risk factors, cigarette smoking is by far the most important in the development of lung cancer. Other risk factors include exposure to certain industrial substances, such as arsenic; some organic chemicals, radon, and asbestos, particularly for persons who smoke; radiation exposure from occupational, medical, and environmental sources; air pollution; tuberculosis; and environmental tobacco smoke in nonsmokers (American Cancer Society, 1998).
Nephrotoxicity is a chemically related risk associated with uranium exposure and has been documented in animal studies at high exposure levels. The kidney is the organ system responsible for excretion of wastes, such as urea, from the blood. In the kidney, more than a million nephrons filter the blood under pressure in the glomerulus and then reabsorb water and selected substances into the blood in the tubules. They also secrete certain solutes from the blood into urine. Water, nitrogenous wastes, and many other substances (excluding colloids, such as proteins that are too large to pass through a healthy glomerulus) pass into the renal tubule, the concentrating and diluting section of the kidney. In the renal tubule (proximal convoluted tubule, Henle's loop, distal convoluted tubule, and collecting duct), most of the substances are reabsorbed into the blood. Thus formed, the urine is conducted from the nephrons via the renal tubules into the ureter, which leads to the bladder.
Uranium internalized in high enough doses is toxic to the kidney (in milligram amounts), but even this exposure resulted in no long-term kidney change. It depresses glomerular function and tubular solute transport. Following inhalation and ingestion of uranium, one may experience no effect, acute but reversible damage to the glomeruli and tubules, or irreparable chronic renal damage, depending on the exposure level.
There are several signs of renal tubular dysfunction and glomerular damage. They include increases in urinary catalase, mild proteinuria, aminoaciduria, and increase in clearance of �-2 microglobulin relative to that of creatinine and altered serum creatinine levels. Routine urine laboratory tests are available to detect these changes. To date, neither renal tubular dysfunction nor glomerular damage has been commonly observed among Gulf War veterans, even among veterans with embedded DU fragments.
There is evidence in humans and animals of repair of damaged tubular epithelial tissue. Acute exposures from accidents have caused acute renal damage, but all exposed persons have recovered with no observable long-term clinical signs or symptoms. The likelihood of developing delayed renal disease from DU exposure if there has been no acute toxicity is not observed or supported by medical evidence. Furthermore, although no formal study has been conducted, evaluations of individuals who worked in DU testing sites across the United States have not shown increased rates of renal failure 15 to 20 years later (Oxenberg, 1998).
Many other conditions are associated with the kidneys but are not associated with nephrotoxicity from uranium exposure or other toxic substances. Examples include kidney stones (nephrolithiasis) and urinary tract infections (pyelonephritis).
Although chronic kidney disease has not been observed in human populations exposed to uranium, kidney disease is common in the United States. More than 20 million Americans suffer from diseases of the kidney and urinary tract. More than 90,000 die each year because of these diseases. About 200,000 Americans suffer from chronic kidney failure and need dialysis to stay alive. Diabetes is the leading cause of chronic kidney failure. It accounts for approximately one-third of new cases of chronic kidney failure in the United States each year. Uncontrolled or poorly controlled high blood pressure is the second leading cause of chronic kidney failure in the United States. It accounts for about 30 percent of all cases. Currently, some 1 million Americans are treated each year for kidney stones. The majority of these cases occur in people between 20 and 40 years of age. Kidney stones are more common in men, who account for about four out of five cases. Approximately 27 million American outpatient visits result from kidney and urinary tract problems (National Kidney Foundation, New York).
The liver is the primary organ in the body responsible for metabolizing impurities and synthesizing proteins essential for life. The liver is also involved in sugar and fat metabolism. When individuals have liver damage, many normal body functions start to break down. Heavy metals have the potential to be toxic to the hepatocytes, the liver cells responsible for the majority of liver function. As these cells die, acute hepatic failure (with profound exposures) may ensue or scarring (cirrhosis) may occur with continued less severe exposures (e.g., chronic alcohol intake or hepatitis).
The liver is an incredibly resilient organ. Acute and chronic insult requires extremely high doses of most toxic substances, including uranium. Animal studies have suggested the possibility of hepatic injury as a result of high doses of uranium. However, liver toxicity has not been a finding among those exposed to DU. Liver toxicity has not been observed in workers at testing sites where individuals were exposed for considerably longer than those serving in the Persian Gulf and not in other occupationally exposed individuals, such as mine workers. In addition, abnormal liver function tests have not been a common finding among individuals who served in the Gulf, making future hepatic disease not likely.[14]
Liver disease is common in the United States but higher-than-expected rates have not been observed in Gulf War veterans. Liver, bile duct, or gallbladder diseases afflict approximately 25 million Americans, or one in every ten. Liver and gallbladder disease is the seventh leading disease-related cause of death. Eliminating alcohol abuse alone could prevent 75-80 percent of the cases of cirrhosis.
Because bone retains uranium, the potential for an increased risk of bone cancer has been explored. As the bone concentration of uranium rises, the committee on the Biological Effects of Ionizing Radiation (NRC, 1988) reports, steadily eating food or drinking water that has uranium in it can be calculated to cause osteosarcoma in humans. The committee reports that the average daily diet of people contains 1 pCi of uranium. The upper limit to the number of bone sarcomas that is calculated to occur is one to two per every 1 million people based on the radiation dose. However, in real life even enriched uranium has not been shown to cause osteosarcomas in people or in animals.
Osteosarcoma is the most common of a number of different types of bone cancer. Bone contains osteoblasts responsible for forming bone matrix (connective tissue and mineral that gives bone its strength) and osteoclasts that prevent too much bone matrix from accumulating. Bone also contains bone marrow. Bone marrow contains fat cells and, most important, hematopoietic cells (the cells that produce blood).
Like osteoblasts of normal bone, the osteosarcomas produce bone matrix. However, the "malignant bone" tissue of an osteosarcoma is not as strong as normal bones. Further osteosarcomas can spread beyond the bone into nearby tissues or through the bloodstream to the lungs, other bones, or other organs of the body.
There are about 900 new cases of osteosarcoma diagnosed in the United States every year. Teenagers are the most commonly affected age group. Osteosarcomas represent about 5 percent of all childhood cancers. In the adolescent years, only leukemias, brain tumors, and lymphomas occur more commonly than osteosarcoma. About 25 percent to 30 percent of patients diagnosed with localized high-grade osteosarcoma will die from the disease. Osteosarcoma is almost twice as common in males as in females. There appears to be a threshold level of radionuclides associated with induction of osteosarcoma. The large study of radium dial painters showed that no osteosarcomas were observed below a dose of about 1,000 rads (10 Gray) (Rowland et al., 1978).
Patients treated with radiation for another cancer have a higher risk of later developing postradiation osteosarcoma. Being treated at a younger age and/or being treated with higher doses of radiation both increase the risk of developing osteosarcoma. There is minimal if any danger of developing osteosarcoma from having diagnostic X rays.
The risk of osteosarcoma clearly increases with radiation dose. However, the amount of radiation emitted by DU is insufficient to raise the risk of osteosarcoma. This is particularly true in adults, whose risk is already quite low (osteosarcomas are more common when bones are growing rapidly, such as in adolescents). Continuous intake of natural dietary uranium (about 35 mBq or 1.4 �g/day) results in a skeletal alpha dose of 0.25 �Gy/year (25 �rad/year) in the United States (Fisenne et al., 1988). Osteosarcoma has never been observed in humans at a skeletal dose less than about 10 Gy and has not been observed in populations exposed to any form of uranium, including enriched uranium.
Information also exists regarding the potential impact continued exposure to elevated uranium levels may have on reproductive health. As with other discussions in this report, consideration must be given to both the radioactive and heavy-metal toxicity associated with exposure. Reproductive health effects can be divided conceptually into structural effects (those damaging or destroying the reproductive system including the germ lines and supportive structures), genotoxic effects (those that alter genetic material that would be passed to offspring, leading either to fetal demise or birth defects), and developmental effects (those affecting the existing fetus, adversely impacting in utero development).
Studies that address human reproductive effects of uranium are limited, and most of the discussions concern exposure to high levels of high-specific-activity uranium, or natural uranium in combination with other sources with the potential to impact reproductive health (e.g., miners exposed to radon and tobacco products). Uranium is primarily an alpha-emitting source of radiation; there is therefore, the potential for DNA damage and fragmentation (ATSDR, 1997, Chapter 2). However, in a report prepared for the U.S. Public Health Service, the Research Triangle Institute was unable to unequivocally identify genetic effects in humans exposed to any radiation level. The study concludes, "because the specific activities of natural and depleted uranium are low, no radiological health hazard is expected from exposure to natural and depleted uranium." (ATSDR, 1997.)
Much effort has been expended in the continuous follow-up of Japanese atomic-bomb survivors, with particular emphasis on genetic and teratogenic effects. The average exposure to the follow-up population of 40,000 persons was 0.30 Sv (30 rem). No statistically significant effects of parental exposure have been found. The atomic-bomb study accounted for the genetically significant dose, the sample size, the sensitivity of the end points, i.e., untoward pregnancy outcomes, sex chromosomal anomalies, survival through the first 17 years of life, and the frequency of biochemical variants (UNSCEAR, 1986). Their conclusion, noting the absence of significant genetic effects in the Hiroshima and Nagasaki populations, is consistent with the expectations based on mouse data (UNSCEAR, 1986).
No genetic or teratogenic effects have been observed from natural background radiation. The genetically significant radiation dose to the gonads from DU in veterans, even with the highest exposure, is increased negligibly above the natural background. For Gulf War veterans, this increase is less than would be expected to occur naturally for individuals living in areas where uranium is more abundant in food and water sources (see Chapter Two). Therefore, no significant genetic effects due to radiation from DU would be expected in Gulf War veterans.
From a toxicological perspective, the uranyl ion, however, is reactive and complexes with organic and inorganic compounds to form chemical substances with the potential to damage DNA. Studies document the impact of mutagenic compounds on reproductive health. Information regarding the explicit impact uranium has on reproductive function in animals varies. Table 2.6 lists various studies and their reported results. Most studies do not demonstrate structural damage at the histologic level; however, some forms of uranium, particularly uranyl nitrate, have been shown to be genotoxic in in vitro settings (Lin, 1993).
Table 2.6
Reproduction Studies Examining the Effects of Uranium on Reproductive Health
| Study | Study Design | Results |
| Domingo et al., 1989 | Natural Uranyl acetate dihydrate Pregnant Mice Administration: Gavage Impact: Developmental |
5, 10, 25, 50 mg/kg/day Material toxicity Decreased fetal body weight/length Malformations at 25 at 50 mg/kg/day Some fetotoxicity at 25 mg/kg/day No embryolethality |
| Hu et al., 1990 | Enriched UO2F2 Male Mice Intraperitoneal Impact: Genotoxicity |
2 mg UO2F2/kg 0.5-6.0 mg/kg (dosing study) Dose relationship DNA elution and UO2F2 |
| Malenchenko, 1978 | Natural UO2 (NO3)6�H2O Rats Subcutaneous (acute) Oral (chronic) Impact: Structural |
Acute: 0.1 mg/kg x 5 days (SQ) Chronic 0.1% uranyl nitrate in water x 4 month Autoimmune appearing thyroiditis and orchitis |
| Llobet, 1991 | Natural Uranyl acetate dihydrate Male Mice Oral (in water) Impact: Structural |
0, 10, 20, 40, 80 mg/kg/day x 64 days No signs of clinical toxicity Decreased sperm counts at all levels Body weights unchanged Histopathologic alterations in 80 mg group Focal tubular atrophy and interstitial cell alterations occur |
| Lin et al., 1993 | Uranyl nitrate In vitro Chinese Hamster Ovaries Impact: Genotoxicity |
0.01-0.30 mM concentration range Dose-related response 50% inhibition of viability at 0.049 mM Decreased cell cycle kinetics Increased micronuclei and chromosomal aberrations Uranyl nitrate is genotoxic and cytotoxic |
| Paternain, 1989 | Uranyl acetate dihydrate Oral Mice Impact: Genotoxicity, Developmental |
0, 5, 10, 25 mg/kg/day x 60 days (males) and through mating/breeding (females) No adverse effect on fertility Embryo lethality at 25 mg/kg/day levels Slow offspring growth |
| Benson and Pellmar, 1998 | DU Pellets Embedded Rats Impact: No effect on structural, genotoxic, or developmental aspects of reproductive health |
0 to 32 1 mm diameter, 2 mm long pellets Significant placental and fetal accumulation No effect on maternal or litter parameters |
| McClain, 1998 | DU Pellets Embedded Female Rats Impact: Developmental |
0 to 32 1 mm diameter, 2 mm long pellets Uranium accumulates in the placenta and fetus Decreased litter size with breeding at 6 months post-implantation |
| Brandom, 1978 | Miners (men) Peripheral blood lymphocytes Impact: Genotoxicity |
Increased chromosome aberrations |
| Dupree, 1987 | Uranium processors (white men) Mortality Impact: Possibly Genotoxicity |
Increased death from all causes, laryngeal carcinoma, circulatory and atherosclerosis, respiratory diseases, pneumonia |
Preliminary results by AFRRI (McClain, 1998) suggest that, at least in animal models, there may be some effects on reproduction (litter size) when DU is implanted into female rats at high levels, particularly when breeding occurred several months after implantation (i.e., some delayed effect). Results suggest that litter size decreases when female rats have implanted with 32 DU pellets (1 mm diameter x 2 mm long). This effect was not noted in female rats implanted with from 4 to 20 pellets for litters right after implantation (Benson and Pellmar, 1998).
With respect to the studies shown in Table 2.6, the concentrations of uranium used to elicit any observed effects through ingestion or inhalation are orders of magnitude greater than the highest exposure that would occur in military or industrial settings (see Table 2.7). To attain the same concentration in blood as in the Swiss mice experiments (Paternain, 1989; Llobet, 1991), grams of uranium would have to be inhaled daily. This quantity was clearly not achievable in the Gulf War setting.
Calculated Blood Uranium Concentrations in Animal Experiments with Mice Versus an Equivalent Human Inhalation During the Gulf War (mg/kg body weight/day)
| MOUSE | ||
| Ingested dose (mg/kg/day) | Ingested per day (mg) | Blood (dose x 0.05) (mg/kg/day) |
| 5 | 0.15 | 0.25 |
| 10 | 0.30 | 0.50 |
| 25 | 0.75 | 1.25 |
| HUMAN | ||
| Inhaled dose (mg/kg/day) | Inhaled per day (mg) | Blood (dose x 0.01) mg/kg/day |
| 25 | 1,750 | 0.25 |
| 50 | 3,500 | 0.50 |
| 125 | 8,750 | 1.25 |
NOTE: Calculations are based on a human male weighing 70 kg and a mouse weighing 0.03 kg. The mouse experiments described above were designed to understand reliable standards for drinking water for humans. Their conclusions were that no toxic effect of any kind could be detected in humans with a drinking water standard of 100 �g per liter (Paternain 1989; Llobet, 1991).
Similarly, for the AFRRI studies employing the use of embedded uranium pellets, the lowest dose to which rats were exposed is reported greater than the highest possible DU levels observed in Persian Gulf veterans with embedded DU fragments (McClain, 1998). Findings also demonstrate the presence of DU in the placenta and the fetus, although the concentration of DU in the fetus is about one order of magnitude less than in the placenta. This result suggests that, while the placenta-fetal barrier protects the developing fetus somewhat from DU, that protection is far less than absolute. No animal studies were found that studied the reproductive effect of uranium on male rats. However, the AFRRI is planning to perform such a study on male animals exposed to DU.
Studies are currently under way by McDiarmid and colleagues at the Baltimore VA Medical Center as part of the DU Follow-Up Program to further evaluate the 33 Gulf War veterans. About half of these 33 individuals have embedded DU fragments. Findings to date have failed to demonstrate clinically relevant abnormalities (beyond those associated with the specific injury). However, most of the veterans with embedded fragments, as previously discussed, continue to have elevations in urinary uranium excretion. VA researchers have also found DU in the semen of some of the veterans with embedded fragments. To date, approximately 17 children have been born to female partners of these 33 men. All of these offspring are without evidence of birth defects (McDiarmid, 1998b). Investigators are completing a follow-up of these individuals, comparing additional laboratory parameters (e.g., sperm morphology and activity, semen characteristics, and serum hormone levels) relative to a control group who did not have uranium exposure (McDiarmid and Keogh, 1998). To date, these investigators have observed normal sperm morphology and counts. Furthermore, examination for chromosomal aberrations and sister chromatid exchange were within the reference limits. Therefore, increased chromosomal aberrations that were reported among uranium miners (Brandom, 1978) have not been found to date among Persian Gulf veterans with embedded DU. There are too few offspring among the 33 veterans with possible embedded DU to draw any conclusions similar to those observed with respect to sex ratio differences in uranium miners' offspring (M[Yuml]ller, 1967).
In summary, to the extent that reproductive health issues related to uranium have been investigated to date, there have not been findings that would suggest a relationship between levels of exposures that could have occurred in the Persian Gulf and those that are associated with adverse outcomes in animal experiments. Further studies are currently under way, particularly with respect to evaluating the impact of uranium on male reproductive health.
[1]Reasons: The direct determination of MPC did not measure a steady-state concentration level, but was based on postmortem analyses, which summarized a range of studies on patients (Hursh and Spoor, 1973). The results may, therefore, bear little relationship to the concentration at which damage would first become overt in otherwise healthy individuals. It further ignores the likelihood that, as with other metals, the damaged kidney may lose uranium at an accelerated rate. Nor does the estimate recognize the fact that renal uranium appears to be present in more than one kinetic pool. The anatomical, morphological, and chemical definition of these uranium pools, or their toxicological significance, remain to be elucidated. Further, distribution of uranium between such pools may be affected by the rate and duration of exposure and other factors. Indeed, the distribution of some heavy metals in the body is known to be influenced by total dose and the rate and route of administration (Aungst, 1981). This presumably is due to the nonlinearity of the processes of metal absorption, distribution, and excretion. Possible changes in compartmentation of uranium in the body as a function of dose and rate, route of its administration, and the possible influence of compartmentation on toxicity have not systematically been explored. Finally, the suggested MPC of uranium ignores the possibility of repair and acquired tolerance. In work with experimental animals, renal change following exposure to uranium has frequently been observed at levels well below the guidance figure of 3 �g per gram (Foulkes, 1990). Present AFRRI studies are also finding acquired tolerance and no adverse renal effects in rats exposed to high levels of DU. The ACGIH today uses threshold limiting value (TLV) for the maximum permissible concentration for inhaled uranium (ACGIH, 1993).
[2]Deposition of the uranyl and other heavy-metal ions in bone is believed to involve competition with calcium for reaction with superficial phosphate groups. Some heavy-metal ions also replace calcium in the crystal structure of bone (Voegtlin and Hodge 1949; Hursh and Spoor, 1973).
[3]The published data are reported in various units, particularly the skeletal values. The reported concentration units are in micrograms or mBq, per unit wet weight, per unit dry weight, per cubic centimeter of wet bone, per unit ash weight, or per gram of calcium. In some cases it is not stated whether the bone marrow was removed. It is of value to convert across the various units to compare data, and data for different bone types. It is necessary to convert to activity per unit wet bone when estimating radiation doses to cells on bone surfaces, the targets for carcinogenesis. The following data apply for osseous tissue (i.e., bone with normal water content in hydroxyapatite plus the collagen matrix) (Fisenne et al., 1988).
Fa = calcium content of bone ash (0.37 g Ca/g ash measured, value for all bone types)
Fap = Calcium in hydroxyapatite (0.40 g Ca/g hydroxyapatite (Ca10(OH)2(PO4)6)
Fdb = Fraction of hydroxyapatite in dried (marrow-free) bone. Measured values include 0.57 for rib, vertebra; 0.70 for femoral shaft (Woodard, 1962)
Fw = Gram dry osseous tissue per gram wet osseous tissue (0.86 for rib, 0.88 for femur) (Gong et al., 1965)
Fd = Mass density of osseous tissue (1.92 g/cm3 for rib and vertebrae, 1.99 g/cm3 for femoral shaft) (Gong et al., 1965)
The adult male and female skeletons contain 5.0 and 4.0 kg of osseous tissue, respectively (ICRP, 1975).
[4]The trend of uranium in air has been observed from 1978 to 1993 (Stevenson and Pan, 1996). The EPA has monitored total isotopic uranium in air in 25 U.S. cities in their Environmental Radiation Ambient Monitoring Program (ERAMS). It is interesting to note that there is a noticeable reduction in the ERAMS uranium air concentration measurements at six of these cities by a factor of four over the period from 1978 to 1993 (from 4 to 1 �Bq/m3). The reason for this is not known; however, the reduction may be associated with lower use of certain fuel types, such as coal. Some airborne uranium is of volcanic origin; eruptions such as Mount St. Helens threw enormous amounts of matter into the stratosphere. This component would be diminishing at the same rate as that observed for nuclear weapons fallout, or about a five- to six-year half-life in the stratosphere.
One of the ERAMS sites, Lynchburg, Virginia, showed a significant increase in the 234U/238U ratio as well as the total uranium activity (about 9 �Bq/m3 versus the normal 1 to 3 �Bq/m3). The addition of a maximum of 1 percent enriched uranium from a local nuclear fuel facility was postulated.
[5]Data calculations use data in Table 4 times 365 days. Total annual intake calculation: 14 mBq/year = [1.22 mBq/day from 238U + 0.019 mBq/day from 234U + 0.0007 mBq/day from 235U] x 365 days/year.
[6]About 10 percent of the amount inhaled is solubilized and goes to the blood where it is excreted or deposited in the kidney, liver, other organs, and the skeleton.
[7]In autopsy results, excess uranium in organs of highly exposed occupational workers (inhaling 40 to 50 mg of uranium) has been measured as long as 38 years after exposure (Kathren and Moore, 1986).
[8]In spite of extensive work on the mechanism of intestinal absorption of heavy metals, no consensus has been reached in this field. The problem was recently reviewed by Foulkes (1995). No adequate evidence has been adduced for the participation of specific and saturable carrier systems in the process, and a possible role for carriers responsible for absorption of physiologically important metals, such as zinc or calcium, in the uptake also of nonessential and toxic heavy metals remains under discussion. A plausible model has been formulated for the whole process: It consists first of the electrostatic binding of the metal to the brush border membrane, followed by its internalization across the membrane at a rate determined by membrane fluidity. The role of membrane fluidity accounts for the temperature dependence of the process, while apparent saturability reflects neutralization of anionic membrane charges. This model satisfactorily accounts for the absorption of cadmium, but its applicability to uranium has not been explored.
[9]Backup material that refers to natural uranium in tissue and diet is included in Tables 2.1, 2.4, and 2.5.
[10]In addition, three Abrams tanks were intentionally destroyed to avoid enemy capture.
[11]As of the date of this report, the findings of much of this important research conducted by the DU Follow-Up Program have not yet been published. The information provided here should therefore be considered preliminary. The information here comes primarily from briefings by Dr. McDiarmid, conference abstracts, and secondary sources.
[12]The AFRRI work is ongoing and the results presented here are preliminary. The preliminary information in this paper comes from an abstract and poster session at the Conference on Federally Sponsored Gulf War Veterans' Illness Research, July 17-19, 1998, or as part of briefings. A paper by Pellmar et al. has been accepted for publication by Toxicological Sciences.
[13]Developing a clinical practice guideline for any condition is a lengthy and involved process and is beyond the scope of this study.
[14]The common blood tests for hepatocyte damage include elevated alanine aminotransferase (ACT or SGPT), aspartate aminotransferase (AST or SGOT), and bilirubin. Damage to the biliary system will produce elevated alkaline phosphatase and gamma glutamyltranspeptidase (GGT). With extensive liver damage, evidence of failure of the liver's synthetic capacity emerges with decreased production of albumin and other serum proteins. This results in ascites, decreased clotting factors resulting in bleeding tendencies, and decreased capacity to metabolize sugars, which in turn results in impaired glucose tolerance or diabetes.
Previous Chapter
Next Chapter
Contents
| First Page | Prev Page | Next Page | Back to
Text |