 )--The fraction of the number of atoms of a radioactive
nuclide that decay in unit time (see Disintegration Constant).
)--The fraction of the number of atoms of a radioactive
nuclide that decay in unit time (see Disintegration Constant).Absorbed Fraction--A term used in internal dosimetry. It is that fraction of the photon energy (emitted within a specified volume of material) that is absorbed by the volume. The absorbed fraction depends on the source distribution, the photon energy, and the size, shape, and composition of the volume.
Absorption--The process by which radiation imparts some or all of its energy to any material through which it passes.
Activity--The number of nuclear transformations occurring in a given quantity of material per unit time (see curie, becquerel).
Activity Median Aerodynamic Diameter (AMAD)--The diameter of a unit-density sphere with the same terminal settling velocity in air as that of the aerosol particle whose activity is the median for the entire aerosol.
Acute Radiation Syndrome--The symptoms that, taken together, characterize a person suffering from the effects of intense radiation. The effects occur within hours or days.
Alpha particle--A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, but of nuclear origin. It comprises two neutrons and two protons and has a mass number of 4 and an electrostatic charge of +2.
Alpha Track--The track of ionized atoms left in any matter by an alpha particle that has traveled through the matter.
Atom--The smallest particle of an element that cannot be divided or broken up by chemical means. It consists of a central core called the nucleus, which contains protons and neutrons and an outer shell of electrons.
Atomic Mass (u)--The mass of a neutral atom of a nuclide, usually expressed in terms of "atomic mass units." The "atomic mass unit" is one-twelfth the mass of one neutral atom of carbon-12; equivalent to 1.6604 x 10-24 g.
Atomic Number--The number of protons in the nucleus of a neutral atom of a nuclide. The "effective atomic number" is calculated from the composition and atomic numbers of a compound or mixture. An element of this atomic number would interact with photons in the same way as the compound or mixture (Symbol: Z).
Atomic Mass Number--See Mass Number.
Atomic Weight--The weighted mean of the masses of the neutral atoms of an element expressed in atomic mass units.
Background Radiation--The amount of radiation to which a member of the general population is exposed from natural sources, such as terrestrial radiation from naturally occurring radionuclides in the soil, cosmic radiation originating from outer space, and naturally occurring radionuclides deposited in the human body.
Becquerel (Bq)--International System of Units unit of activity and equals that quantity of radioactive material in which one transformation (disintegration) occurs per second (1 Bq = 1 disintegration per second = 2.7 x 10-11 Ci).
Beta Particle--Charged particle emitted from the nucleus of an atom. A beta particle has a mass and charge equal in magnitude to that of the electron. The charge may be either +1 or -1.
Biological Half-Time--The time required for a biological system, such as that of a human, to eliminate by natural process half of the amount of a substance (such as a chemical substance or radioactive material) that has entered it.
Body burden, Chemical--The total amount of a substance found in a biological system, such as that of a human.
Body burden, Radioactivity--The amount of radioactive material present in the total body.
Bone Seeker--Any compound or ion that migrates in the body and preferentially deposits into bone.
Carcinogen--A chemical capable of inducing cancer.
Carcinoma--Malignant neoplasm composed of epithelial cells, regardless of their derivation.
Charged Particle--An ion. An elementary particle carrying a positive or negative charge.
Collective dose--The sum of the individual doses received in a given period of time by a specified population from exposure to a specified source of radiation.
Committed Effective Dose (S)--Following an intake into the body of a radioactive material there is a period during which the material gives rise to an effective dose. The committed effective dose is the time integral of the effective dose rate. If the time interval is not specified it is implied that the value is 50 years for the adult.
Committed Equivalent Dose Ht(t)--Following an intake into the body of a radioactive material there is a period of time during which the material gives rise to an equivalent dose. The committed equivalent dose is the time integral of the equivalent dose rate. If the time interval is not specified it is implied that the value is 50 years for the adult.
Contamination, Radioactive--Deposition of radioactive material in any place where it is not desired.
Cosmic Rays--High-energy particulate and electromagnetic radiation, which originate outside the earth�s atmosphere.
Curie (Ci)--A unit of radioactivity. One curie equals that quantity of radioactive material in which there are 3.7 x 1010 nuclear transformations per second (1 Ci = 3.7 x 1010 disintegrations per second = 3.7 x 1010 Bq). The activity of 1 gram of radium is approximately 1 Ci.
Daughter Products--See Progeny.
Decay, Radioactive--Transformation of the nucleus of an unstable nuclide by spontaneous emission of charged particles and/or photons (see Disintegration).
Decay Chain or Decay Series--A sequence of radioactive decays (transformations) beginning with one nucleus. The initial nucleus, the parent, decays into a daughter nucleus that differs from the first by whatever particles were emitted during the decay. If further decays take place, the subsequent nuclei are also usually called daughters or progeny. Sometimes, to distinguish the sequence, the daughter of the first daughter is called the granddaughter, etc.
Decay Constant ( )--The fraction of the number of atoms of a radioactive
nuclide that decay in unit time (see Disintegration Constant).
)--The fraction of the number of atoms of a radioactive
nuclide that decay in unit time (see Disintegration Constant).
Decay Product, Daughter Product--A new isotope formed as a result of radioactive decay. A nuclide resulting from the radioactive transformation of a radionuclide, formed either directly or as the result of successive transfor mations in a radioactive series. A decay product (daughter product) may be either radioactive or stable.
Depleted uranium--Uranium having a percentage of 235U smaller than the 0.7 percent found in natural uranium. It is obtained as a by-product from uranium isotope separation (see Enrichment).
Developmental Toxicity--The occurrence of adverse effects on the developing organism that may result from exposure to a chemical prior to conception (either parent), during prenatal development, or postnatally to the time of sexual maturation. Adverse developmental effects may be detected at any point in the life span of the organism.
Disintegration Constant--The fraction of the number of atoms of a radioactive
nuclide which decay in unit time;  is the symbol for the decay
constant in the equation N = Noe-
is the symbol for the decay
constant in the equation N = Noe- t, where
No is the initial number of atoms present, and N is the number of atoms
present after some time (t) (see Decay Constant).
t, where
No is the initial number of atoms present, and N is the number of atoms
present after some time (t) (see Decay Constant).
Disintegration, Nuclear--A spontaneous nuclear transformation (radioactivity) characterized by the emission of energy and/or mass from the nucleus. When large numbers of nuclei are involved, the process is characterized by a definite half-life (see Transformation, Nuclear).
Dose Assessment--An estimate of the radiation dose to an individual or a population group usually by means of predictive modeling techniques, sometimes supplemented by the results of measurement.
Dose, Effective--The effective dose is the equivalent dose (Ht) multiplied by a tissue-weighting factor, wt, with the special name Sievert (Sv). The tissue-weighting factor represents the contribution of the organ or tissue to the total detriment due to the effect resulting from uniform irradiation of the body. E = (wr)(wt)(D)
The sum of the weighted equivalent doses in all the tissues and organs in the body.
It is given by E = 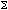 WtHt where
Wt is the weighting factor for tissue T.
WtHt where
Wt is the weighting factor for tissue T.
Dose, Equivalent (Ht)--The equivalent dose is absorbed dose D multiplied by a radiation-weighting factor Wr to account for the different qualities of radiation (alpha, beta, and gamma) in terms of potential effect. The special name is the Sievert (Sv). The present weighting factors are as follows: alpha radiation is wr = 20; beta and gamma radiation wr = 1. The dose equivalent expresses all radiation on a common risk scale. (The unit of equivalent dose is the rem. In SI units, the equivalent dose is the sievert, which equals 100 rem.)
Dose, Pharmacological--A general term denoting the quantity (mass) of a substance introduced into the body. For special purposes it must be appropriately qualified.
Dose, Radiation Absorbed--The energy imparted to matter by ionizing radiation per unit mass of irradiated material at the place of interest. The unit of absorbed dose is the rad. One rad equals 100 ergs per gram. In SI Units, the absorbed dose is the gray, which is 1 J/kg (see Rad). Absorbed dose rate is the absorbed dose per unit time.
Dose, Radiation Cumulative--The total dose resulting from repeated or continuous exposures to radiation.
Dose Rate--Absorbed dose delivered per unit time.
Dosimetry--Quantification of radiation doses to individuals or populations resulting from specified exposures.
Electron--A stable elementary particle having an electric charge equal to �1.60210 x 10-19 C (coulombs) and a rest mass equal to 9.1091 x 10-31 kg. A positron is a positively charged "electron" (see Positron).
Electron Volt--A unit of energy equivalent to the energy gained by an electron in passing through a potential difference of one volt. Larger multiple units of the electron volt are frequently used: keV for thousand or kilo electron volts, MeV for million or mega electron volts.
Embryotoxicity and Fetotoxicity--Any toxic effect on the conceptus as a result of prenatal exposure to a chemical; the distinguishing feature between the two terms is the stage of development during which the insult occurred. The terms, as used here, include malformations and variations, altered growth, and in utero death.
Enriched Material--(1) Material in which the relative amount of one or more isotopes of a constituent has been increased. (2) Uranium in which the abun dance of the 235U isotope is increased above normal.
Enrichment, Isotopic--An isotopic separation process by which the relative abundance of the isotopes of a given element is altered, thus producing a form of the element that has been enriched in one or more isotopes and depleted in others. In uranium enrichment, the percentage of uranium-235 in natural uranium is increased from 0.7 percent to >90 percent in a gaseous diffusion process based on the different thermal velocities of the constituents of natural uranium (234U, 235U, 238U).
Equilibrium, Radioactive--In a radioactive series, the state that prevails when the ratios between the activities of two or more successive members of the series remains constant.
Secular Equilibrium--If a parent element has a very much longer half-life than the daughters (so there is not appreciable change in its amount in the time interval required for later products to attain equilibrium) then, after equilibrium is reached, equal numbers of atoms of all members of the series disintegrate in unit time. This condition is never exactly attained but is essentially established in such a case as radium and its series to 210Pb. The half-life of radium is about 1,600 years, of radon, approximately 3.82 days, and of each of the subsequent members, a few minutes. After about a month, essentially the equilibrium amount of radon is present; then (and for a long time) all members of the series disintegrate the same number of atoms per unit time. At this time, the activity of the daughter equals the activity of the parent.Exposure (Chemical)--Contact of an organism with a chemical or physical agent. Exposure is quantified as the amount of the agent available at the exchange boundaries of the organism (e.g., skin, lungs, gut) and available for absorption.Transient Equilibrium--If the half-life of the parent is short enough so the quantity present decreases appreciably during the period under consid eration, but is still longer than that of successive members of the series, a stage of equilibrium will be reached after which all members of the series decrease in activity exponentially with the period of the parent. At this time, the ratio of the parent activity to the daughter activity is constant. An example of this is radon (half-life of approximately 3.82 days) and successive members of the series to 210Pb.
Exposure (Radiation)--Being exposed to ionizing radiation or to a radioactive material.
Gamma Ray, Penetrating--Short-wavelength electromagnetic radiation of nuclear origin.
Genetic Effect of Radiation--Inheritable change, chiefly mutations, produced by the absorption of ionizing radiation by germ cells.
Gray (Gy)--SI Unit of absorbed dose, J/kg (1 Gy = 1 J/kg = 100 rad).
Half-Life, Radioactive--Time required for a radioactive substance to lose 50 percent of its activity by decay. Each radionuclide has a unique half-life.
Immunologic Toxicity--The occurrence of adverse effects on the immune system that may result from exposure to environmental agents, such as chemicals.
In vitro--Isolated from the living organism and artificially maintained, as in a test tube.
In vivo--Occurring within the living organism.
Ion--Atomic particle, atom, or chemical radical bearing a net electrical charge, either negative or positive.
Ionization--The process by which a neutral atom or molecule acquires a positive or negative charge.
Ionization Path (Track)--The trail of ion pairs produced by ionizing radiation in its passage through matter.
Ionizing Radiation--Any radiation capable of displacing electrons from atoms or molecules, thereby producing ions. Examples: alpha, beta, gamma, X rays, and neutrons. High doses of ionizing radiation may produce severe skin or tissue/organ damage.
Isobars--Nuclides having the same mass number but different atomic numbers.
Isotopes--Nuclides having the same number of protons in their nuclei, and hence the same atomic number, but differing in the number of neutrons and therefore in the mass number. Almost identical chemical properties exist between isotopes of a particular element. The term should not be used as a synonym for nuclide.
Stable Isotope--A nonradioactive isotope of an element.Linear Energy Transfer (LET)--A measure of the ability of biological material to absorb energy from ionizing radiation; specifically, for charged particles traversing a medium, the energy lost per unit length of path. A similar quantity may be defined for photons.
Low-LET--Energy transfer characteristic of light charged particles such as electrons produced by X- and gamma rays where the distance between ionizing events is large on the scale of a cellular nucleus.Lung Clearance Class (days, D; weeks, W; years, Y)--A classification scheme for inhaled material according to its rate of clearance from the pulmonary region of the lungs to the blood and the gastrointestinal tract. Also used ICRP classes of F (fast), M (medium), and S (slow) clearance.High-LET--Energy transfer characteristic of heavy charged particles, such as protons and alpha particles where the distance between ionizing events is small on the scale of a cellular nucleus.
Average LET--Is specified to even out the effect of a particle that is slowing down near the end of its path and to allow for the fact that secondary particles from photon or fast-neutron beams are not all of the same energy.
Mass Numbers (A)--The number of nucleons (protons and neutrons) in the nucleus of an atom.
Neutron--Elementary nuclear particle with no electric charge equal.
Nucleon--Common name for a constituent particle of the nucleus. Applied to a proton or neutron.
Nuclide--A species of atom characterized by the constitution of its nucleus. The nuclear constitution is specified by the number of protons (Z), number of neutrons (N), and energy content; or, alternatively, by the atomic number (Z), mass number A = (N+Z), and atomic mass. To be regarded as a distinct nuclide, the atom must be capable of existing for a measurable time. Thus, nuclear isomers are separate nuclides, whereas promptly decaying excited nuclear states and unstable intermediates in nuclear reactions are not so considered.
Parent--A radionuclide that, on disintegration, yields a specified nuclide either directly or as a later member of a radioactive series.
Power, Stopping--A measure of the ability of a material to absorb energy from an ionizing particle passing through it; the greater the stopping power, the greater the energy absorbing ability (see Linear Energy Transfer).
Progeny--The decay product or products resulting after a radioactive decay or a series of radioactive decays. The progeny can also be radioactive, and the chain continues until a stable nuclide is formed.
Proton--Elementary nuclear particle with a positive electric charge equal numerically to the charge of the electron and a rest mass of 1.007277 mass units.
Rad (rad)--The unit of absorbed dose equal to 0.01 Gy or J/kg in any medium. (1 rad = 100 erg/g = 0.01 Gy) (see Absorbed Dose).
Radiation--The emission and propagation of energy through space or through a material medium in the form of waves (e.g., the emission and propagation of electromagnetic waves or of sound and elastic waves). The term radiation or radiant energy, when unqualified, usually refers to electromagnetic radiation. Such radiation commonly is classified according to frequency, as microwaves, infrared, visible (light), ultraviolet, and X and gamma rays (see Photon) and, by extension, corpuscular emission, such as alpha and beta radiation, neutrons, or rays of mixed or unknown type, such as cosmic radiation.
Radiation, Background--See Background Radiation.
Radiation, External--Radiation from a source outside the body.
Radiation, Internal--Radiation from a source within the body (as a result of deposition of radionuclides in body tissues).
Radiation, Ionizing--Any electromagnetic or particulate radiation capable of producing ions, directly or indirectly, in its passage through matter (see Radiation).
Radioactivity--Spontaneous nuclear transformations that result in the formation of new elements. These transformations are accomplished by emission of alpha or beta particles from the nucleus or by the capture of an orbital electron. Each of these reactions may or may not be accompanied by a gamma photon.
Radioactivity, Natural--The property of radioactivity exhibited by more than 50 naturally occurring radionuclides.
Radioisotopes--An unstable isotope of an element that decays or disintegrates spontaneously, emitting radiation. Approximately 5,000 natural and artificial radioisotopes have been identified.
Radionuclide--A radioisotope or radioactive nuclide characterized by the constitution of its nucleus.
Reaction (Nuclear)--An induced nuclear disintegration (i.e., a process occurring when a nucleus interacts with a photon, an elementary particle, or another nucleus). In many cases the reaction can be represented by the symbolic equation: X+a-->Y+b or, in abbreviated form, X(a,b) Y. X is the target nucleus, a is the incident particle or photon, b is an emitted particle or photon, and Y is the product nucleus.
Rem (rem)--A unit of equivalent dose. The equivalent dose in rem is numerically equal to the absorbed dose in rad multiplied by the quality factor (radiation-weighting factor) (1 rem = 0.01 sievert).
Reproductive Toxicity--The occurrence of adverse effects on the reproductive system that may result from exposure to a chemical. The toxicity may be directed to the reproductive organs and/or the related endocrine system. The manifestation of such toxicity may be noted as alterations in sexual behavior, fertility, pregnancy outcomes, or modifications in other functions that are dependent on the integrity of this system.
Roentgen--unit of x-radiation or gamma radiation equal to the amount of radiation that produces ionization of either sign equal to one electrostatic unit of charge in one cubic centimeter of dry air at 0 degrees and standard atmospheric pressure.
Short-Term Exposure Limit (STEL)--The maximum concentration to which workers can be exposed for up to 15 min continually. No more than four excursions are allowed per day, and there must be at least 60 min between exposure periods. The daily TLV-TWA may not be exceeded.
SI Units--The International System of Units as defined by the General Conference of Weights and Measures in 1960. These units are generally based on the meter/kilogram/second units, with special quantities for radiation including the becquerel, gray, and sievert.
Sickness, Acute Radiation (Syndrome)--The complex symptoms and signs characterizing the condition resulting from excessive exposure of the whole body (or large part) to ionizing radiation. Five Sv (500 rem) is fatal 50 percent of the time. The earliest of these symptoms are nausea, fatigue, vomiting, and diarrhea and may be followed by loss of hair (epilation), hemorrhage, inflam mation of the mouth and throat, and general loss of energy. In severe cases, where the radiation dose is relatively high, death may occur within two to four weeks. Those who survive six weeks after exposure of a single high dose of radi ation may generally be expected to recover.
Sievert (Sv)--The SI unit of any of the quantities expressed as equivalent or effective dose. The equivalent dose in sieverts is equal to the absorbed dose, in grays, multiplied by the radiation-weighting factor (1 Sv = 100 rem). The effective dose is the equivalent dose multiplied by the tissue-weighting factor.
Specific-activity--Radioactivity per unit mass of a radionuclide.
Target Organ Toxicity--This term covers a broad range of adverse effects on target organs or physiological systems (e.g., renal, cardiovascular) extending from those arising through a single limited exposure to those assumed over a lifetime of exposure to a chemical.
Target Theory (Hit Theory)--A theory explaining some biological effects of radiation on the basis that ionization, occurring in a discrete volume (the target) within the cell, directly causes a lesion that subsequently results in a physiological response to the damage at that location. One, two, or more "hits" (ionizing events within the target) may be necessary to elicit the response.
Teratogen--Any chemical that causes birth defects.
Threshold Limit Value (TLV)--The maximum concentration of a substance to which most workers can be exposed without adverse effect. TLV is a term used exclusively by the American Congress of Governmental Industrial Hygienists (ACGIH). Other terms used to express the same concept are the MAC (maximum allowable concentration) and the OSHA equivalent PEL (permissible exposure limits).
Transformation, Nuclear--The process by which a nuclide is transformed into a different nuclide by absorbing or emitting a particle.
TWA--Time-weighted average.
X rays--Penetrating electromagnetic radiations whose wave lengths are very much shorter than those of visible light. They are usually produced by bombarding a metallic target with fast electrons in a high vacuum. X rays (called characteristic X rays) are also produced when an orbital electron falls from a high energy level to a low energy level.