Many ill veterans report new intolerances to chemicals (Gordon 1997), and some studies are underway to further assess chemical sensitivity in ill PGW veterans (Fiedler, Kipen, et al., 1996).
The lack of a clinical case definition for either MCS or illnesses in PGW veterans complicates examination of a connection between these two phenomena. Nonetheless, several factors are consistent with a connection between illnesses in some PGW veterans, and "toxicant-induced loss of tolerance," or MCS. Similar exposures, namely to acetylcholinesterase-inhibiting agents, characterize ill PGW veterans and many MCS patients. Some work is beginning to suggest mechanisms by which cholinergic exposures, experienced by some PGW veterans, could induce chemical sensitivity. These mechanisms include partial kindling of the limbic system and alteration of nasopharyngeal mucosal function. Studies have found that similar EEG abnormalities may characterize persons with selected AChE inhibition exposures seen during the PGW (sarin, OP pesticides) and persons with MCS, though it has not been shown that ill PGW veterans with consistent symptoms share these EEG abnormalities (neither has it been shown that they do not). SPECT studies (single photon emission computerized tomography, which evaluates regional cerebral blood flow) in a small segment of PGW veterans with chemical sensitivities have reportedly been abnormal, as have SPECT scans in individuals exposed to pesticides (see Chapter Fourteen, "Chronic Effects"), although larger samples of all veterans and controls should be evaluated using blinded testing. At present, it is premature to accept a connection between illnesses in PGW veterans and MCS; however, it is premature to reject the possibility of such a connection. Further study is warranted. The following text explores these issues in greater detail.
For each condition, the following is true:
This chapter will consider the following topics: parallels in exposures and symptoms in ill PGW veterans and patients with MCS; incidence of chemical sensitivities in PGW veterans; results of a SPECT study in a sample of PGW veterans with chemical sensitivities; and points of contact between MCS and PB.
Parallels in Exposures and Symptoms in Ill PGW Veterans and in MCS
Many patients with MCS and ill PGW veterans have in common prior exposures to AChE-inhibiting agents. Subgroups of MCS patients have self-reported exposures to carbamate or OP pesticides or chlorpyrifos (Ziem, 1997), to solvents in industry, or to mixed solvents associated with building remodeling. Carbamate and OP pesticides are AChE inhibitors, and, as previously noted, organic solvents have been shown to inhibit AChE in vitro (Korpela and Tahti, 1986; Korpela, Tahti, et al., 1986). Many PGW veterans experienced exposures to PB, to low levels of nerve agents, pesticides, and solvents, as well as oil fires, vaccines, and perhaps infectious disease.
MCS and PGW patients both report prominent alterations in concentration and memory (Miller, 1994; Fukuda, Nisenbaum, et al., 1998; Iowa Persian Gulf Study Group, 1997). Moreover, MCS and PGW subjects share other categories of symptoms, including musculoskeletal, respiratory, and dermatological. Moreover, as previously mentioned, many ill PGW veterans report new chemical sensitivities (Gordon, 1997).
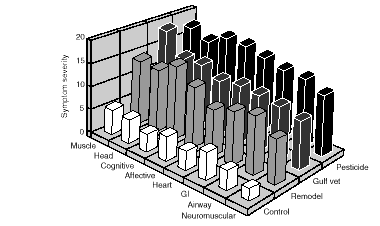
The ten most frequent complaints of PGW veterans enrolled in the Persian Gulf Health Registry with complaint data available are shown in Table 11.1. Symptoms in MCS patients are shown in Table 11.2. These tables are not directly comparable because the mode of questioning influences the symptoms reported. Nonetheless, both lists share fatigue (systemic complaints), headache, skin rash, musculoskeletal complaints, CNS complaints, GI complaints, and respiratory complaints. But ear, nose and throat symptoms, sleep disturbances (unless that is included in systemic complaints), genitourinary, and circulatory symptoms are distinct. MCS subjects report higher levels of these complaints--a difference that could reflect true differences in subjective symptom patterns, differences in exposure levels, or differences in referral and self-referral patterns for the two conditions. Moreover, both similarities and discrepancies in listed symptoms could be artifacts of the methods of questioning. Indeed, one investigator reported, in testimony, quite similar rates of various symptoms in ill PGW veterans and MCS subjects, presumably employing a common mode of questioning (Miller, 1996b). However, these findings have not been published in a peer-reviewed source.
| Complaint | Men | Women |
| Fatigue | 23.3 | 20.7 |
| Headache | 23.0 | 17.7 |
| Skin rash | 18.2 | 18.5 |
| Muscle or joint pain | 14.5 | 16.5 |
| Loss of memory or "other general symptoms" | 13.9 | 14.2 |
| Shortness of breath | 7.6 | 8.0 |
| Sleep disturbances | 5.3 | 5.9 |
| Abdominal pain | 3.9 | 2.5 |
| Other symptoms involving skin and integument | 3.8 | 3.2 |
| Diarrhea and other GI symptoms | 3.6 | 4.5 |
SOURCES: IOM report (Committee to Review the Health Consequences of Service During the Persian Gulf War; IOM, 1996); Persian Gulf Health Registry data provided to IOM Committee to Review the Health Consequences of Service During the Persian Gulf War.
| Symptom | Percentagea |
| Ear-nose-throat | 90-100 |
| GI | 40-95 |
| Systemic | 60-90 |
| Musculoskeletal | 80-100 |
| CNS, excluding headache | 80-85 |
| Headache | 65-100 |
| Dermatologic | 60-80 |
| Lower respiratory | 75-100 |
| Genitourinary | 20-65 |
| Circulatory | 25-80 |
aPercentage ranges reflect prevalence for four MCS subgroups.
SOURCE: Miller and Mitzel, 1995; Davidoff and Keyl, 1996.
EEG abnormalities have been reported in MCS subjects (Miller, 1992) and in persons exposed to AChE inhibitors, such as OPs (Duffy, Burchfiel, et al., 1979; Duffy and Burchfiel, 1980). (Such changes include increased beta and decreased alpha activity.) How similar these changes are in those with different exposures, and whether the EEG changes seen in pesticide-exposed persons with MCS also occur in ill Gulf War veterans with PB exposure, is unknown.
Incidence of Chemical Sensitivities in PGW Veterans
No peer-reviewed data are available regarding the incidence of new chemical sensitivities in PGW veterans. Testimony from many veterans has included comments about new sensitivities to foods, cigarettes, alcohol, and chemicals (Subcommittee on Human Resources, 1997a, 1997b), and in one report, many of 549 PGW veterans evaluated reported "high intolerance" to chemicals in the environment (Gordon, 1997). Moreover, according to evidence presented in testimony, of 59 consecutive PGW veterans seen at the Houston VAMC Regional Referral Center, 78 percent reported new intolerances (Miller, 1996b) (see Table 11.3).[1] While this analysis is severely limited by the absence of a control population, it does suggest possible development of new chemical and food sensitivities in some ill PGW veterans, at a rate that may exceed that in the general population. Consequently, it favors efforts to examine PGW illnesses in the context of efforts to study putative MCS.
| Food or Chemical | Percentage with New Intolerances |
| Chemical inhalants | 78 |
| Medications | 40% of those taking drugs |
| Alcohol | 66% of alcohol users |
| Caffeine | 25% of caffeine users |
| Tobacco use | 74 |
| Foods | 78 |
| Specific foods | 64 |
| Illness after meals | 49 |
SOURCE: Non-peer reviewed testimony (Miller, 1996b).
SPECT Study in PGW Veterans with Chemical Sensitivities
One small study (six cases, six controls) performed SPECT scanning (a method for looking at regional cerebral blood flow) on six male PGW veterans "with chemical sensitivities" and six controls reportedly determined not to have toxic exposures. Abnormalities were reported in all six SPECT reports from PGW veterans with sensitivities (abnormalities were classified as mild in one, moderate in two, and severe in three), while all six reports from controls were read as normal. Soft tissue diversion was noted in three cases (but no controls), lobar discrepancies in three cases (and one control), focal findings in five cases (and one control), and phase mismatching in four cases (and one control). Findings in all PGW veterans were noted to be similar to those seen in patients with known exposure to widely recognized neurotoxins "including petroleum distillates and pesticides" (Simon, Hickey, et al., 1994). (Indeed, qualitative and quantitative SPECT performed on patients with OP pesticide and solvent exposures have demonstrated abnormalities despite nondiagnostic MRI brain scans--abnormalities that are reportedly distinct from the findings seen with depression and "late-life chronic fatigue syndrome." (Heuser, Mena, et al., 1994).) SPECT is regarded by some as a "sensitive and potent" indicator of CNS function impairment after neurotoxic exposure (Heuser, Mena, et al., 1994). However, specificity remains an issue, because SPECT abnormalities may occur in many conditions, including depression. Of note, it has been suggested that focal cortical hypoperfusion with limited temporal lobe involvement may suggest a direct cortical effect of neurotoxins, rather than a limbic effect suggested in the kindling hypothesis.
The reported SPECT findings in PGW veterans may have important implications if they can be replicated in a larger, more carefully controlled study, but the present study has significant limitations. The sample was extremely small; subject selection procedures, including criteria for "chemical sensitivity," were not clearly delineated; the SPECT readings were qualitative and not stated to be blinded; no primary outcome variable was identified; and no statistics were offered. Moreover, results in all veterans with chemical sensitivities might not be reflective of results in other ill veterans; the estimate of 78 percent of ill veterans with new sensitivities, cited previously, might not reflect values in larger samples of veterans or ill veterans, and criteria for chemical sensitivity may differ from those in the present study. Therefore, these findings, while intriguing, must be viewed as preliminary. Nonetheless, attempts should be made to replicate the finding of SPECT abnormalities in the form of focal hypoperfusion and to extend this work by ascertaining if these defects are selectively enhanced when patients are symptomatic following reexposure to an offending chemical, to determine whether SPECT scanning could offer a much needed, if costly, objective marker for chemical sensitivity.
Points of Contact Between MCS and PB
Because a clinical case definition has not been accepted for either MCS or PGW illnesses, any discussion of common etiology must be regarded as hypothesis-generating rather than hypothesis-supporting. Nonetheless, there are points of contact between cholinergic function (which is influenced by PB) and the putative MCS syndrome, and these points of contact merit review:
At least 250,000 PGW veterans were exposed to AChE inhibition through PB. An estimated 100,000 may have been exposed to low levels of nerve agent following the demolition of the Iraqi Khamisiyah ammunitions depot (Gulflink, 1997), and many additional veterans were exposed to pesticides, solvents, and petroleum products. Thus, AChE exposure is common to many PGW veterans and to many or most MCS subjects.
In one study, 10 of 10 MCS subjects were found to have abnormal rhinolaryngoscopic findings including edema, excess mucus, "cobblestoning" (an alteration in the appearance of the mucosa), mucosal injection (redness of the mucosa from surface capillaries), and blanching around vessels (Meggs and Cleveland, 1993). However, there were no controls and no blinding in evaluation.
A relation to PB could conceivably occur, since cholinergic function is involved in nasopharyngeal mucociliary action (Sastry and Sadavongvivad, 1979). Therefore dysregulation of cholinergic function (which may be influenced by PB alone or in concert with other exposures influencing the cholinergic system) could prolong nasal exposure to chemicals and perhaps participate in altering nasal resistance and contributing to symptoms. As a related or independent mechanism, some lipophilic pesticides and other agents could partition into membranes of the respiratory mucosa, altering their properties (Moya-Quiles, Munoz-Delgado, et al., 1995).[4] This mechanism would not require central exposure to AChE inhibition.
Animal studies suggest that priming an animal with high or repeated concentrations of any of various chemicals, including pesticides (Bell, Miller, et al., 1992), and subsequently reexposing the animal to low concentrations of the same or different chemicals may produce increased likelihood of paroxysmal electrical discharge in the amygdala. Though the agents used to sensitize animals may differ chemically, the effects on the limbic system are quite similar. Bokina (1976) has suggested that these findings parallel clinical observations in MCS and that kindling could amplify reactivity to low-level inhaled and ingested chemicals and initiate persistent affective, cognitive, and somatic symptomatology (Bell, Miller, et al., 1992). Partial kindling (kindling leading to levels of paroxysmal electrical discharge below those resulting in seizure activity) has been shown to increase avoidant behavior in animals, including cats (Bell, Schwartz, et al., 1993a). One could speculate that MCS results from entrainment of strong aversive signaling in response to chemical exposures. (Of note, vagal stimulation has been approved by the FDA for treatment for intractable partial seizures (Zoler, 1998), potentially consistent with a connection between the ACh system and seizures, but the nature of the relationship remains confusing.)
The amygdala is closely connected to the hippocampus, another brain area involved in limbic functioning that is also felt to play an important role in learning and memory (in which many MCS and PGW subjects report impairments). Hippocampal damage may affect production, storage, or release of excitatory and inhibitory neurotransmitters, and small perturbations in hippocampal function can have lasting effects on behavior and cognition. The hypothalamus, part of the brain with rich limbic input that controls many autonomic and somatic functions, has also been postulated to play a role in development of symptoms in patients with subjective sensitivities (Miller, 1992).
Factors that lower the seizure threshold (that is, factors that facilitate the development of seizures in response to potentially seizure-producing stimuli), such as estrogen in women, might be expected to facilitate kindling sensitization (Bell, Schwartz, et al., 1993a).[5]
Indeed, studies in rats have found a greater susceptibility to sensitization in female than male rats (Antelman, 1988); and sex differences have been found for other, analogous neuronal effects (such as long-term facilitation following a single exposure to amphetamine) (Robinson, Becker, et al., 1982). This finding may have a clinical correlation in that predominantly females report symptoms of MCS (Miller and Mitzel, 1995), though the MCS cases that follow certain incitants are more commonly male (such as exposure to industrial solvents), reflecting the predominantly male group that is exposed. Self-reported illness from foods and chemicals in young adults (ascertained with no attempt to meet MCS "criteria") also occurs predominantly in females (Bell, Schwartz, et al., 1993b). Meanwhile, although 93 percent of PGW veterans were male, and while there may be a slight trend toward higher reported incidences of symptoms in female veterans, differences, if any, are not marked. Among those veterans in the VA Persian Gulf Health Registry, 69 percent of women reported their health as all right, good, or very good compared with 73 percent of men (Committee to Review the Health Consequences of Service During the Persian Gulf War; IOM, 1996). These subjects were self-selected to participate in the registry, and the generalizability to all PGW veterans is uncertain. (Of note, exposures for male and female veterans may have differed. If male veterans experienced more exposures then females, then symptom rates may underrepresent differences in response to exposures. No data regarding differences in exposures between males and females have been identified.)
Genetic polymorphism and quantitative variability in many enzymes involved in detoxification of xenobiotics offer a possible mechanism by which individual differences in susceptibility could be examined (see Chapter Eight, "Individual Differences in Reactions to PB"). Moreover, interactions with other drugs, exposures, or stresses may influence the effective dose received by an individual (see Chapter Nine, "Interactions Between PB and Other Exposures"). Interactions between chemicals may produce an effect in other ways, perhaps by targeting the same or different elements of the nervous system. It has been observed that less than 10 percent of the 70,000 commercially available chemicals have been evaluated for neurotoxicity. Furthermore, data regarding effects of such chemicals are almost universally deficient in information about chronic or long-latency effects (Landrigan, Graham, et al., 1994).
Cacosmia (abnormal perception of smells as bad) is found to be at best weakly related to such psychological variables as trait shyness (r = 0.18), anxiety (r = 0.08), and depression (r = 0.16) (Bell, Schwartz, et al., 1993b). Moreover, MCS subjects do not differ significantly from controls in factors thought to predict psychiatric illness, such as family psychiatric history, treatment of a psychiatric condition not linked to illness, or unusually intense or long-lasting stress during childhood (Davidoff and Keyl, 1996). Though overall psychiatric symptoms are greater in MCS patients than in controls (correlating significantly with the presence of illness) (Simon, Daniell, et al., 1993; Davidoff and Keyl, 1996), this may reflect effect rather than cause. Similar studies could profitably be undertaken in PGW veterans: a negative result in a well-designed study would reduce the likelihood that illness is of "psychological" origin. A positive result would not necessarily discriminate between organic and "psychological" origin.
It must be remembered that some mood disorders (for instance) are linked to abnormalities in ACh system function. Persons with altered ACh function could have different susceptibility to effects from drugs like PB that act on the ACh system: thus, if a relation between development of illnesses in PGW veterans and prior mood disorder (or other conditions known to be associated with altered ACh function) were found, this would not necessarily imply increased likelihood of a "psychosomatic" origin to disease (if such a concept has any meaning at all); rather, if susceptibility to illnesses in PGW veterans is found to be differential according to psychiatric predisposing factors and existing conditions, the relevant neurochemistry of those conditions should be assessed for clues to the biological basis of illnesses in PGW veterans (see Chapter Thirteen, "Neurotransmitter Dysregulation").
Several observations have been cited to suggest that MCS and illnesses in PGW veterans could have an organic basis. These include the following:
(Claims that stress or "psychogenic illness" is substantially responsible for illnesses in PGW veterans can and should be subjected to the same minimal scrutiny. That is, the fraction of ill subjects who have various symptoms following other stressful circumstances would be expected to be similar to the fractions with the same symptoms among PGW veterans for the stress hypothesis to be supported. To the extent this is untrue, the stress hypothesis is weakened. No attempt to verify quantitative comparability of symptom frequencies in illness following major stressors has been identified.)
As noted previously, Miller (1996b) reported quantitatively similar ordering of symptoms (using eight symptom scales derived by factor analysis) in 59 consecutive ill PGW veterans (of whom 78 percent reported new onset chemical sensitivities since the war) as in 37 pesticide-exposed civilians with chemical sensitivities, in non-peer reviewed testimony (Miller, 1996b). These results were quite different from those in normal controls. If ranking of symptoms referable to different organ systems are indeed confirmed to be similar in PGW veterans and MCS patients, this strengthens the case for operation of a similar mechanism for illnesses in some PGW veterans.

SOURCE: Miller (1996a)
Although the connection between MCS and illnesses in PGW veterans is supported only by limited evidence, enough suggestive evidence is present to warrant further scientific study.
Many veterans are known to have been exposed to AChE-inhibiting chemicals (including PB, pesticides, and low-level nerve agents), which some feel may predispose to subjective chemical sensitivities.
Compatible Illness
Unpublished, non-peer reviewed research reports new chemical sensitivities in a high fraction (78 percent) of a small number (59) of tested ill veterans. Unpublished, non-peer reviewed research finds that ranking and frequency of symptoms is similar in these veterans and in non-PGW patients with subjective chemical sensitivities following self-reported exposures. No peer-reviewed evidence is yet available to support or refute these findings.
Compatible Link Between Exposure and Illness
No published effort has been made to link presence of "compatible" exposures to new subjective chemical sensitivities in PGW veterans.
[2]C-fiber neurons are nerve cells that branch extensively in the mucosa and contain neuropeptides, or small proteins that serve to convey signals, such as "vasoactive intestinal peptide," "substance P," which is involved in perception of pain and production of inflammation, and calcitonin gene-related peptide.
[3]One cross-sectional survey, cited in a review only as "personal communication," reportedly found a relationship between self-reported mucosal symptoms in the workplace, such as eye, nose, throat, and respiratory irritation, and self-described heightened chemical sensitivity to such workplace elements as tobacco, fumes from a photocopying machine, new carpet, pesticides, new furniture, or paint (Bascom, 1994).
[4]Of anecdotal interest, some patients with self-described chemical sensitivities report noticeable symptom abatement with behaviors that increase salivation such as chewing gum; however, abatement of symptoms through this means does not necessarily imply that the pathology involved is nasopharyngeal.
[5]Consistent with the identified effect of estrogen on seizures in animals, recent studies in humans have found that epileptic women have fewer seizures after menopause, and hormone replacement treatment may worsen seizures (Harden, 1997). The effect of estrogen on enhancing ACh function or action is suggested by postmenopausal estrogen's ability to delay onset of dementia (Jacobs, Tang, et al., 1998)--Alzheimer's-type dementia involves ACh dysfunction and is treated by drugs that increase ACh action. However, complicating the question of how ACh influences seizures, evidence has recently shown that stimulation of the vagus nerve--which produces ACh action in the periphery--has been shown to significantly reduce the number of seizures (Handforth, 1998). Of incidental note, headache may occur as a preseizure event (French 1997), suggesting a possible role for increased ACh action in some headaches. Headaches are a prominent symptom in many ill PGW veterans.
[6]Distress at being labeled with psychogenic illness has been voiced by both MCS patients (Miller, 1994) and by PGW veterans (Subcommittee on Human Resources, 1997a, 1997b; Zeller, 1997). Such characterizations have engendered alienation among veterans, who have referred to them as degrading and have correctly described them as unfounded (Sumpter-Loebig, 1997).