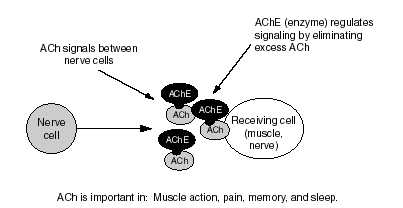
Figure S.1--How Normal Neurotransmission Works
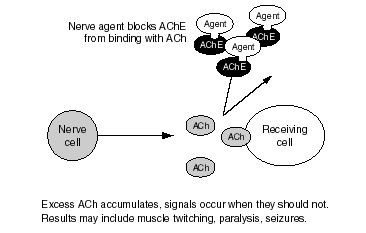
Figure S.2--Nerve Agent Blocks AChE Enzyme
PB is used primarily to protect troops against attack by one particular nerve agent, soman. During the PGW, Iraq was known to have nerve agents, including sarin, and had weaponized them by putting them into rockets, bombs, and missile warheads. While it was not known whether Iraq had militarized the nerve agent soman, it was known that the former Soviet Union had soman, and there were concerns, particularly since the fragmentation of the former Soviet Union, that Iraq may have purchased soman. Iraq used chemical weapons against Iran and the Kurds. Because of the possibility that Iraq had soman, coalition troops were provided with PB, to be used for protection when the threat of chemical warfare was deemed high. Evidence from that time and subsequent to the PGW suggests that Iraq had weaponized the nerve agents sarin, cyclosarin, and perhaps tabun and VX, but no evidence uncovered suggests they had soman or had weaponized it.
This report examines issues surrounding the safety and to a lesser degree the effectiveness of PB. The sections on safety consider seven hypotheses of how PB might lead to negative health effects. Each hypothesis is investigated to determine if it can be rejected as a possible causal factor. If sufficient evidence cannot be marshaled to rule out a hypothesis, this does not imply that it is necessarily a causal factor, only that the possibility cannot be dismissed.

Figure S.1--How Normal Neurotransmission Works

Figure S.2--Nerve Agent Blocks AChE Enzyme
For most nerve agents, postexposure treatment confers adequate protection from death with amounts of nerve agent that are presumed likely in warfare. The postexposure treatments in use by the military are atropine and pralidoxime (also called "2PAM"). Atropine antagonizes (blocks) the effects of ACh at one type of receptor, and pralidoxime pulls the nerve agent off the AChE, restoring the action of AChE to normal. In addition to PB, troops were given three "Mark I" kits containing injections of both atropine and pralidoxime for use after a nerve agent attack (Army and possibly Marines) or were given individual injectors of these agents (Air Force and Navy).
Unfortunately, in the case of soman, a reaction termed "aging" takes place in the nerve agent-AChE complex within only minutes of exposure. Once this reaction has taken place, pralidoxime can no longer pull the nerve agent off the AChE molecule. Thus, troops would not have enough time to administer pralidoxime before AChE is permanently inactivated, which could ultimately result in death. Aging also happens with other nerve agents, but it takes hours to occur after sarin, cyclosarin, tabun, or VX exposure, which allows troops adequate time to administer pralidoxime before aging has taken place, helping to restore AChE action. Animal evidence suggests that to ensure adequate protection against death in the event of a soman attack, PB pretreatment must be employed.[1]
PB acts--it is thought--by reversibly binding to (and, incidentally, inhibiting) the AChE on the site where the nerve agent would bind, thus blocking soman from permanently inactivating the AChE (see Figure S.3). As soman is cleared from the body, PB spontaneously leaves the AChE and restores functional AChE. The dose of PB used by troops, 30 mg each eight hours, is chosen to inhibit 20 to 40 percent of the AChE. The goal is to ensure that at least this proportion of AChE is relatively safe from permanent inactivation in case of exposure to soman, while allowing enough residual AChE activity (60-80 percent) to prevent significant side effects and to allow personnel to adequately carry out their functions. It is believed that to protect most troops from death by amounts of soman that might realistically occur in a combat setting, a person must be able to withstand approximately five times the normal lethal dose. This level of protection has not been achieved with postexposure treatments alone (that is, with atropine and pralidoxime) but requires use of PB as a pretreatment adjunct in tests in nonhuman primates.
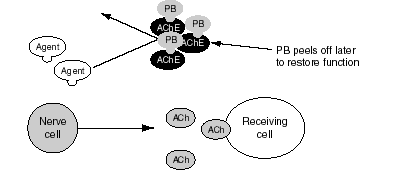
Figure S.3--PB Prevents Nerve Agent from Binding to AChE Enzyme
In monkeys and to a lesser extent in other animals, PB protects against the lethal effects of the nerve agent soman; but it does not prevent severe incapacitation of the animals from high doses of the nerve agent. So even if data signifying protection in primates at higher doses of PB do extrapolate to humans at lower doses, troops are likely to be incapacitated in the presence of a soman attack. Moreover, in animal studies, PB appears to reduce somewhat the protection (conferred by postexposure atropine and pralidoxime) against lethal effects of some other nerve agents, such as sarin and cyclosarin. This apparent reduction in protection still provides for high protection in some animals (with "protective ratios," characterizing protection against lethal effects, that are still several times higher than the fivefold protection that has been designated as desirable). However, no direct evidence ensures that the increased vulnerability to death (reduced protection) that PB may bring for such nerve agents as sarin leaves high or "adequate" (fivefold) protection intact in humans. Again, substantial interspecies differences have been seen, with changes not only in magnitude but also in the sign (direction) of the effect of PB, and testing of protection by PB against lethal effects of nerve agents in humans cannot, of course, be done.
PB is normally largely excluded from entry to the brain by the "blood-brain barrier," which bars access to the brain of many chemicals and organisms that circulate in the blood. If PB gains entry to the brain, adverse effects can result from the binding of PB to ACh receptors in the brain. These effects may include confusion, emotional changes such as depression, sleep alterations, and difficulties with concentration and memory.
This report explores whether PB--with this panoply of acute effects--could plausibly have contributed to chronic symptoms reported by ill PGW veterans. Far higher doses of PB, used for far longer times (typically lifelong) have been employed for decades to treat patients with myasthenia gravis, and this has been assumed by many to indicate that lower-dose, briefer use in nerve agent pretreatment will be safe. However, data from patients with myasthenia might not extrapolate completely to those taking PB for other purposes. For one thing, PB is used in patients with myasthenia gravis to restore nicotinic cholinergic function (at least in the muscles) toward normal. In those without myasthenia gravis, PB raises ACh function away from normal. Thus, extrapolating evidence of safety from patients with myasthenia gravis is somewhat analogous to assuming that, since high doses of insulin are tolerated--or even necessary--in some patients with diabetes (to bring their blood sugar toward normal), therefore a smaller dose of insulin should surely be safe in those without diabetes. We know this is not the case and that smaller doses of insulin given to normal individuals can cause adverse effects and even death. There are other important reasons PB may not be safe for nonmyasthenic individuals, which are discussed later.
Hypotheses regarding heightened susceptibility to effects of PB include the following:
The permeability of the "blood-brain barrier" in PGW veterans may have been enhanced due to stress and other conditions of war, permitting increased access of PB to the brain. Moreover, PB itself may increase the access of other agents to the brain. Data demonstrating breach of the blood-brain barrier, consequently allowing increased access of PB to the brain in conditions of stress, comes from recent limited research conducted on rodents. However, human data suggest a possible increase in central nervous system (CNS) side effects of PB during the war compared to peacetime, which could also reflect increased access of PB to the brain during stressful circumstances.
The degree to which the blood-brain barrier may have been compromised in conditions of stress may influence the possible contribution of several other hypotheses. For example, dysregulation of the brain's cholinergic system is less likely to result from PB use unless PB gains access to the brain--or other AChE inhibitors do so, perhaps facilitated by PB. (In fact, however, changes in cholinergic function occurring in the periphery could have central consequences).
Individual Differences: Do Physiologic Differences Influence Susceptibility to PB?
Individual differences in susceptibility may also contribute to a connection between PB and chronic illnesses. How is it, if PB is a contributor to chronic illnesses in PGW veterans, that some PGW veterans who took PB became ill, while others who took a similar amount did not? Individual differences of many kinds play a role in the effect of PB on the body. First, differences occurred in the dose of PB actually taken by troops. Moreover, different absorption of PB pills from the gut into the blood; differences in chemical structure, in efficiency of action, and in available amounts of enzymes that clear PB from the blood; and differences in other factors all may lead to different PB blood levels. Furthermore, differences in AChE inhibition occur even for the same blood level of PB. Finally, differences in toxic effects may occur even if individuals experience the same degree of AChE inhibition, perhaps reflecting individual differences at baseline in elements of the complex ACh system.
Altogether, these factors provide substantial opportunity for differences in effect from the "same" oral dose of PB from one individual to another. From a clinical standpoint, individual differences in acute susceptibility to PB obviously occur and are reflected in differences in side effects individuals experienced in response to PB. There is limited evidence that the acute susceptibility differences may arise from mechanisms relevant to differences in chronic symptoms in PGW veterans--one study finds a relation between certain chronic illness "syndromes" in ill PGW veterans and self-reported adverse acute response to administration of PB. If PB is a contributor to chronic illnesses in some PGW veterans, then individual differences in susceptibility could play a role in determining which individuals are affected.
Interactions with Other Exposures: Do Interactions Between PB and Other Exposures Enhance the Toxicity of Effects?
Another factor that may play a role in the connection between PB and illnesses in PGW veterans involves possible interactions between PB and other exposures. Animal studies indicate that additive or even synergistic toxicity--that is, toxic effects from a group of chemicals that are more than the sum of the toxic effects from the individual chemicals--may occur with PB and other exposures that some veterans may have experienced. These may include pesticides and insect repellents, as well as caffeine, perhaps nerve agents, and chemicals released by the body itself in conditions of stress.
The degree to which these interactions between PB and other exposures may play a role in PGW veterans is unclear for several reasons. First, we do not have good data regarding who received which exposures, complicating any epidemiological studies to determine the effect of these interactions. Second, the data from animal studies are difficult to extrapolate to PGW veterans because extremely high doses of both PB and the interactants have been used in studies in animals--doses many times higher than those experienced by PGW veterans.
Addressing the question of whether important synergistic effects would occur with lower doses of interactants--more comparable to those administered to PGW veterans--is not simple. There is no good way to assess whether low doses in animals produce effects comparable to those reported by ill veterans. In the existing animal studies, relatively crude measures, such as gross incoordination in walking, or death, are often employed because it is difficult to test animals for more-subtle effects. If lower doses are studied, more-sensitive measures will need to be found. Nonetheless, because evidence of synergistic toxicity exists, interactions between PB and other agents or exposures remain a possible avenue by which increased effect or toxicity of PB may have occurred in some veterans.
Bromism is a condition characterized by neurological and psychiatric symptoms and caused by the accumulation of bromide in the body. It has been suggested that PB administration during the PGW may have led to this condition. However, bromism emerges as an unlikely cause of chronic illness, because the cumulative doses of bromide ingested in PB pills by most PGW veterans were too small to cause bromism, and the time-course of illness in many ill PGW veterans is too long to be typical of this condition, which usually abates within days to months of discontinuing exposure to bromide. Although it is conceivable that bromism could have contributed to illnesses for some rare veterans with special circumstances, bromism is highly unlikely as a significant contributor to illnesses in most ill veterans.
Multiple Chemical Sensitivity (MCS): Does PB Lead to MCS?
MCS is a putative symptom complex involving multiple self-reported "sensitivities" or adverse subjective responses to low levels of a host of apparently unrelated foods and chemicals. Symptoms may include headaches, difficulty concentrating, memory impairment, and musculoskeletal and abdominal complaints. MCS is not universally accepted as a syndrome by scientists or clinicians. It lacks a widely accepted case definition, and no objective technique has been identified to distinguish those who report symptoms from those who do not. Since MCS itself is not universally accepted or well understood, it is poorly positioned to explain illnesses in PGW veterans.
Still, there are several intriguing similarities. First, symptoms reported by patients with MCS are not confined to chemical sensitivities; and other symptoms, such as musculoskeletal symptoms and headaches, are reportedly similar to those described by ill PGW veterans. Second, some ill PGW veterans report that they have developed new chemical sensitivities since their return from the PGW. Third, many or most ill PGW veterans and MCS patients experienced exposures to AChE-inhibiting drugs or chemicals prior to developing their symptoms. Moreover, the genesis of MCS has been proposed to relate to excessive ACh activity, or reduced AChE activity, which may presumably have been experienced by PGW veterans exposed to PB. At present, because of limitations noted above, MCS cannot serve as an explanation for illnesses in any PGW veterans. However, it can be hoped that ongoing research into each condition (MCS and illnesses in PGW veterans) will advance understanding of possible cholinergic mechanisms for both, whether or not these conditions are found to converge.
Neuromuscular Junction (NMJ) Effects: Does PB Produce NMJ Changes?
Nerves signal to muscles using ACh at the neuromuscular junction (NMJ), and this signaling causes the muscles to contract. Administration of high doses of AChE-inhibiting drugs, such as PB, has been shown in animals to produce destructive changes to the muscle tissue and to produce pre- and postsynaptic changes in the NMJ--that is, changes that occur both at the side of the signal-sending nerve cell and at the side of the signal-receiving muscle cell. These changes begin after a single dose of PB. Though some destructive effects begin to recede even if use of PB is continued, partially restoring the appearance of the muscle and of the NMJ, this restoration has not been complete in all cases, even long after administration of PB has been stopped. Thus, chronic--and perhaps permanent--changes take place.
Findings at the NMJ are important for two reasons. First, some of the symptoms reported by PGW veterans include musculoskeletal problems and fatigue, to which the effects of PB at the NMJ might contribute. Second, the NMJ is the most accessible cholinergic synapse, and it is therefore the easiest one to study. Researchers have hoped that effects evident at the NMJ will accurately reflect effects at acetylcholinergic synapses in the brain. In some instances, but not others, this hope has been borne out. Additional and different processes play important roles in brain synapses.
Neurotransmitter Dysregulation: Does PB Alter Regulation of Neurotransmitters, Particularly ACh?
Abnormal regulation of neurotransmitter systems may occur following the administration of drugs that act on these systems. "Downregulation," in this case the (hypothesized) attenuation or suppression of the acetylcholinergic system following use of such AChE-inhibiting drugs as PB, is an instance of dysregulation. That is, during and after PB use, effects may occur that counteract the abnormally high activity of ACh induced by PB. Changes consistent with downregulation have been demonstrated in the NMJ with drugs like PB. Moreover, some evidence suggests that dysregulation changes may also occur in the brain, when AChE-inhibiting chemicals gain access to it. These changes have been demonstrated in animals, using AChE inhibitors that readily gain access to the CNS, and typically at doses that achieve higher levels of AChE inhibition than expected for doses to which veterans were exposed. These may include both changes that enhance and that depress ACh action, with different effects occurring for different components of the ACh system and in different parts of the brain. Different effects may also occur with widely differing time-scales, from very brief to long-term or perhaps permanent. They may involve changes in production, packaging, and release of the neurotransmitter; changes in the number of receptors for ACh, in the "affinity" of these receptors for ACh (the avidity with which ACh attaches), and in their response to ACh; and changes in production and degradation of the enzymes that regulate breakdown of ACh.
By hypothesis, symptoms described by PGW veterans could be manifestations of a prolonged dysregulation effect from PB use. But this hypothesis has not been directly substantiated by data. If PB gains access to the brain, discontinuing PB exposure might lead to symptoms of low (or altered) ACh activity. However, little basic science has been done to characterize the time-course of dysregulation changes, and more needs to be understood about the doses and the durations of use that might produce it--recalling that individual differences are surely at play. Clinically, ACh is known to play an important role in memory, sleep, and pain, as well as muscle action, and the most prominent symptoms reported by PGW veterans include problems with memory, sleep, pain, and fatigue. Moreover, studies have been done in which drugs that boost ACh function, particularly nicotinic function, have specifically benefited memory, pain, fatigue, diarrhea, and sleep apnea. (Sleep apnea is a specific sleep abnormality that has been reported among ill PGW veterans). These findings, indicating the selective benefit of ACh-enhancing drugs for problems that figure prominently in complaints of ill PGW veterans, are consistent with the possibility that these symptoms in PGW veterans could derive from ACh downregulation (or, more generally, dysregulation) resulting from use of PB. However, they are not proof of this hypothesis. In addition, these studies showing benefit to these symptoms from ACh-enhancing drugs have not been done in ill PGW veterans, and it is unknown whether ill veterans would derive similar benefit. At present, the idea of neurotransmitter dysregulation as an explanation for illnesses in some PGW veterans is speculative. Research is needed to clarify what role, if any, such dysregulation might have in the development of chronic symptoms.
Chronic Effects
Some literature suggests the possibility of chronic effects by AChE inhibitors generally, including PB. Data regarding chronic effects, particularly from low-dose exposures that do not produce acute symptoms, are meager and studies are frequently of poor quality. Some studies fail to demonstrate such abnormalities on neuropsychological or other tests in persons with prior AChE exposures. Other studies report chronic changes in nerve and muscle function, EEGs, regional cerebral blood flow, or neuropsychological tests, typically following exposure to AChE-inhibiting pesticides or to nerve agents.
Still other studies have evaluated whether ill PGW veterans indeed have chronic neurological abnormalities. The findings of these studies have been mixed. Differences in findings may reflect both the strategy for selecting ill veterans and the character of the tests performed. If chronic effects are present, they could be missed by failing to properly identify cases and controls or by performing tests that are not sensitive to the specific deficits that ill veterans may have. Of course, if chronic neuropsychological effects are not present in PGW veterans more often than in others, then neither PB nor any other exposure will need to be invoked as an explanation.
A few small studies of chronic neuropsychological findings in ill PGW veterans suggest that selected ill veterans have statistically lower scores on neuropsychological tests than do healthy controls. Although it appears that some ill veterans do have mildly diminished neurocognitive function, the extent to which an excess number of veterans do so remains to be clarified. The reductions in function that have been observed do not appear to relate to one or a small number of neurocognitive abilities. However, since the acetylcholinergic system plays a prominent role in many functions of the brain, abnormalities resulting from the disruption of the ACh system might be expected to span many functions. An additional important issue is whether such impairment, if present, is related to use of--or an adverse response to--PB. One study suggested a connection between adverse acute response to PB and current neuropsychological syndromes in Gulf War veterans. Moreover, a recent study from Britain found that self-report of exposure to PB was strongly and significantly linked to current CDC-defined Gulf War illness among British veterans. These and other completed works are limited by the use of self-reporting to determine exposure to PB. Individuals who are ill may remember use of PB differently from individuals who are not ill. (Self-report appears to be the best gauge of use available because records of who received PB, who took PB, and how much they took, were not maintained. Moreover, in the British study, risk ratios were not materially different for troops for whom records were available to confirm risk-factor status, compared to the group as a whole, suggesting against a major role for recall bias.) In short, there is suggestive evidence that some AChE inhibitors may cause chronic neurological changes. There is some objective evidence that chronic neurological changes exist in some ill PGW veterans compared with healthy controls. There is limited evidence that development of some types of chronic neuropsychological changes may be linked to acute response to administration of PB. Consequently, one cannot rule out the possibility that long-term effects of PB might occur and might participate in the production of neuropsychological and other deficits reported by some PGW veterans.
Other Effects
PB's effects on hormones, sleep, the serotonergic and other neurotransmitter systems, and the observation of increased deaths from accidents in PGW veterans after the war may merit additional study. Many PGW veterans report difficulties with sleep. Sleep is prominently regulated by the ACh and serotonin/melatonin systems, both of which might be influenced by PB. Sleep apnea may be particularly common in ill PGW veterans, and some studies outside the PGW population suggest that sleep apnea may respond to nicotine (a "nicotinic" acetylcholinergic agent), consistent with proposed dysregulation of the ACh system in ill PGW veterans. PB may mimic serotonin, providing another avenue for association between PB use and sleep difficulties in PGW veterans. Disruption of sleep, in turn, has been shown to have a role in some pain syndromes. Moreover, sleep disruption is strongly linked to susceptibility to motor vehicle accidents, and epidemiologic studies show an increase in death by motor vehicle accidents in PGW veterans. (Other neurological characteristics that some researchers are investigating in subsets of ill PGW veterans may also dispose them to increased risk of accidental death, perhaps independent of sleep difficulties. For instance, abnormalities in eye movement coordination if confirmed could retard reaction times, which could translate to increased risk when at the wheel.)
Several issues important to military use of PB were reviewed but are not discussed in detail in this report, including data regarding the efficacy of PB as a nerve agent pretreatment adjunct, data on acute physiological and performance effects of PB, and information about acute side effects outside the warfare setting. (Limited information on the acute effects of PB is included in Appendix B.) While important to the future military use of PB, these data do not directly address the development of chronic illnesses in PGW veterans.
Concern regarding PB as a possible source of chronic symptoms is relatively new, and research in this area is in its infancy. Human data regarding chronic effects are mostly observational, and these epidemiological studies are complicated by lack of a consistent clinical case definition distinguishing which PGW veterans should be counted as ill or as neurologically symptomatic as a result of their involvement in the PGW. The lack of good data regarding who received which exposures hinders study as well. When both the exposure and the outcome are not well characterized, it is doubly difficult to evaluate clearly the connection between PB exposure and an adverse outcome. While some experimental data related to short-term PB effects are available from studies using non-war volunteers, such studies have not looked at long-term effects and have not entailed conditions of high stress and interactions with other exposures that may have conditioned susceptibility to PB in the PGW. Most experimental studies relating to toxic effects, and involving stress and drug interactions, are done in animals at relatively high doses, and the degree to which this evidence extrapolates to humans is unknown.
The findings reported here, in which it is concluded that PB cannot be excluded as a contributor to illness in PGW veterans, differ from conclusions of some prior investigating bodies, such as the Presidential Advisory Committee and the Institute of Medicine. Three significant factors contribute to these differences. First, a more extensive literature review, and particularly a more in-depth examination of the ACh system, has been performed. Second, the approach to evaluation of evidence differs. Some prior reports appear to have interpreted the evidence as though absence of proof that PB contributed to illness constitutes proof that it did not. Finally, new evidence has become available that provides additional rationale for concern regarding PB--evidence not available to previous groups. Similarly, our own findings are provisional and subject to change as new evidence emerges.
Second, uncertainties remain concerning the effectiveness of PB in protection of humans against nerve agents. Most data on effectiveness of PB in primates derive from studies using higher doses, and how well these extrapolate to lower dose use in humans remains ambiguous. Finally, some literature, again mostly based on animal studies, indicates that use of PB may reduce somewhat the effectiveness of postexposure treatment for some nonsoman nerve agents. The extent and importance this reduction would have in humans is unknown.
These findings based on the scientific literature raise many questions and have important implications relating to the use of PB in military deployments. Clearly, substantially more research into the effectiveness of PB for humans is needed--and quickly. Meanwhile, the issue is a complex one, involving trading off uncertain health risks--but risks now shown to be biologically plausible--against uncertain gains from use of PB in the warfare setting.
[2]Source citations for other references in the "Summary" can be found in the corresponding chapters of the main body of this report.