Organophosphate (OP) compounds were first synthesized in significant quantities during the 1940s, when tetraethylpyrophosphate was developed as an insecticide.
Azamethiphos
General Information. Azamethiphos is an OP pesticide that was probably procured locally during the PGW as a fly bait. While there is no EPA registration number for azamethiphos, it has been used in Canada, Scandinavia, the United Kingdom, and France to control sea lice infestations in fish farms. Azamethiphos is also available in Mexico, primarily for fly control. Commercially available azamethiphos products outside the United States include Alfacron 10 and Snip.
Alfacron 10 contains 10 percent azamethiphos as the active ingredient and is used as a wettable powder. A thick paste is obtained by mixing 200 mL of tepid water with 250 g of Alfacron 10, which will cover either 100 m2 of floor space or 200 m2 of walls. Alfacron can also be applied by spraying or painting with a liquid solution of 500 g Alfacron 10 in 4 L of tepid water. This mixture will cover 50 m2 of floor space or 100 m2 of wall surfaces. Snip is a 1 percent azamethiphos fly bait that contains Z-9 tricosene (female housefly pheromone), which attracts flies to eat the granular bait. The recommended application is 200 g of Snip spread on a 100 m2 space frequented by flies. The bait becomes more effective if the space has been previously wetted with water or milk.
The chemical identity of azamethiphos is shown in Table 7.1, and Table 7.2 summarizes its physical and chemical properties.
Table 7.1
Chemical Identity of Azamethiphos
| Characteristic | Information |
| Chemical class | Organophosphate |
| Chemical name | Phosphorothioic acid, S-{[6chloro-2-oxooxazolo(4,5b)pyridin-3-(2H)-yl]methyl} O,O-dimethyl ester |
| Trade names | Alfacron 10; Snip Fly Bait |
| Chemical formula | C9H10ClN2O5PS |
| CAS Registry number | 35575-96-3 |
Table 7.2
Physical and Chemical Properties of Azamethiphos
| Property | Information |
| Molecular weight | 324.68 |
| Color/form | Orange yellow granules or gray to white crystalline powder |
| Water solubility at 20°C | 0.11 mg/mL (soluble in organic solvents such as methylene chloride, benzene, methanol, hexane, and n-octanol) |
| EPA toxicity classification | Class III |
| ACGIH TLV-TWA | NA |
| NIOSH REL-TWA | NA |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | NA |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | NA |
| EPA | NA |
| IARC | NA |
|
NA = not available |
|
Availability and Recommended Use of Azamethiphos During ODS/DS. Both Alfacron 10, a wettable powder, and Snip, a granular bait, were reported to have been used during the PGW, and both were probably obtained locally (OSAGWI, personal communication). No NSN exists for azamethiphos-containing products.
Chlorpyrifos
General Information. Chlorpyrifos is a broad-spectrum insecticide originally used primarily to kill mosquitoes, although it is no longer registered for this use. It is registered for a variety of uses and sites and is effective in controlling cutworms, corn root worms, cockroaches, grubs, flies, termites, fire ants, and lice. Chlorpyrifos acts primarily as a contact poison, with some action as a systemic poison. It is available in a variety of formulations, including granules, wettable powder, dustable powder, and emulsifiable concentrate.
The chemical identity of chlorpyrifos is shown in Table 7.3, and Table 7.4 summarizes its physical and chemical properties.
Availability and Recommended Use of Chlorpyrifos During ODS/DS. The chlorpyrifos products shipped to the Persian Gulf during ODS/DS are shown in Table 7.5.
Table 7.3
Chemical Identity of Chlorpyrifos
| Characteristic | Information |
| Chemical class | Organophosphate |
| Chemical name |
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate |
| Trade names | Brodan, Detmol UA, DMS-0971, Dowco 179, Dursban, Empire, ENT-27, 311, Eradex, Lorsban, Pageant, Piridane, Pyrinex, Scout, Stipend |
| Chemical formula | C9H11Cl3NO3PS |
| CAS Registry number | 2921-88-2 |
Table 7.4
Physical and Chemical Properties of Chlorpyrifos
| Property | Information |
| Molecular weight | 350.62 |
| Color/form | White to tan crystalline solid with amber oil |
| Odor | Mild sulfur/mercaptan |
| Water solubility at 25°C | 2 mg/L |
| Partition coefficient (Kow) | 9.1 x 104 |
| Soil sorption coefficient (Koc) | 6,070 |
| Vapor pressure at 20°C | 1.87 x 10-5 mm Hg |
| EPA toxicity classification | Class II |
| ACGIH TLV-TWA | 0.2 mg/m3 (skin) |
| NIOSH REL-TWA | 0.2 mg/m3 (skin) |
| NIOSH REL-STEL | 0.6 mg/m3 (skin) |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | 3 x 10-3 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | A4 |
| EPA | NA |
| IARC | NA |
|
NA = not available. |
|
Table 7.5
Formulations of Chlorpyrifos Available During ODS/DS
|
NSN |
Name |
Form |
(%) |
Unit Size |
Application Method |
| 6840-00-402-5411 | Dursban 4E | Liquid | 40.8-42.8 | 5-gal can |
|
| 6840-01-203-6161 | Dursban 1.5 ULV | Liquid | 19.36 | 5-gal can |
|
| 6840-01-210-3392 | Dursban L.O. | Liquid | 42 | 40-mL bottle |
|
|
Source: Provided by OSAGWI. |
|||||
Environmental Characteristics of Chlorpyrifos. Due to its strong affinity for organic soils, chlorpyrifos adsorbs strongly to soils and sediments, and leaching and runoff are not significant. Adsorbed chlorpyrifos degrades under UV light, via chemical hydrolysis, and by the action of soil microbes. The soil half-life of chlorpyrifos ranges from two weeks to more than one year, depending on soil texture, soil pH, and climate. When applied to moist soils, chlorpyrifos has a volatility half-life of 45 to 163 hours, with 62 to 89 percent of the application remaining on the soil after 36 hours (Kamrin, 1997).
Chlorpyrifos Residues. Perhaps most important for estimating human exposures are studies of the fate of chlorpyrifos following its application. In one representative study of chlorpyrifos residues following application for residential termite control, ambient air samples and floor wipe samples were taken for seven days after either broadcast or total-release aerosol applications (Lu, 1998). The ambient air samples were taken at a height of one meter. The entire study was conducted with no ventilation and again when the area was ventilated with forced air for 30 minutes immediately after application. The results are summarized in Table 7.6. Peak air concentrations of 0.118 mg/m3 (broadcast) and 0.082 mg/m3 (aerosol) were measured four hours after application. More chlorpyrifos was deposited on the carpet floors by broadcast applications than by aerosol applications. Residues on non-target surfaces (e.g., furniture) were greater with aerosol applications than with broadcast applications. Similar decay rates were observed in other studies (Gurunathan, 1998), and similar residue fractions were measured in studies conducted in homes and offices (Fenske, 1990; Currie, 1990).
Table 7.6
Air and Surface Chlorpyrifos Residues Following Residential Broadcast and Aerosol Applications
|
Chlorpyrifos Deposited |
|||||||||
|
Broadcast Application |
Aerosol Application |
||||||||
|
Vent On |
Vent Off |
Vent On |
Vent Off |
||||||
|
Days After Application |
(mg/m3) |
(�g/cm2) |
(mg/m3) |
(�g/cm2) |
(mg/m3) |
(�g/cm2) |
(mg/m3) |
(�g/cm2) |
|
1 |
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
Source: Compiled from Lu (1998). |
|||||||||
Diazinon
General Information. Diazinon is an insecticide used to control cockroaches, silverfish, ants, and fleas in residential, non-food-preparation buildings. It is used as a bait to control scavenger yellow jackets in the western United States. Diazinon is also commonly used in home gardens and on farms to control a wide variety of sucking and leaf-eating insects. It is available in dust, granules, seed dressings, wettable powder, and emulsifiable solution formulations.
The chemical identity of diazinon is shown in Table 7.7, and Table 7.8 summarizes its physical and chemical properties.
Availability and Recommended Use of Diazinon During ODS/DS. The diazinon formulations that were shipped to the Persian Gulf during ODS/DS are listed in Table 7.9.
Table 7.7
Chemical Identity of Diazinon
| Characteristic |
|
| Chemical class | Organophosphate |
| Chemical name | O,O-diethyl 0-[6-methyl-2-(1-methylethyl-4-pyrimidinyl) ester |
| Trade names | G-24480, Antigal, Basudin, Diazol, D-z-n, Garden Tox, HelfaCat, HelfaDog, Neocidol, Parasitex, Sarolex, Spectracide, Taberdog, Tabercat |
| Chemical formula | C12H21N2O3PS |
| CAS Registry number | 333-41-5 |
Environmental Characteristics of Diazinon. Diazinon has low persistence in soil, with a half-life of two to four weeks. It seldom migrates below the top half-inch of soil, but in some instances it may contaminate groundwater. The breakdown in water depends on acidity: At high acidic levels, diazinon has a half-life of 12 hours, while in neutral solution the half-life can reach six months (Kamrin, 1997).
Diazinon Residues. Airborne and surface concentrations of diazinon were measured after broadcast spray application on the floors of offices. A 1 percent solution of diazinon in water was applied at approximately 0.03 L/m2 of floor surface. The airborne diazinon concentration peaked four hours after application, at 0.16 mg/m3, and it remained at nearly the threshold limit value 24 hours after application when rooms were unvented. Surface concentrations of diazinon were highest at 48 hours after application, 38 ng/cm2 (Currie, 1990).
Table 7.8
Physical and Chemical Properties of Diazinon
| Property | Information |
| Molecular weight | 304.36 |
| Color | Colorless (technical grade); pale yellow to dark brown |
| Odor | Faint ester-like odor |
| Water solubility at 25°C | 40 mg/L |
| Soil sorption coefficient (Koc) | 6.45 x 10+3 |
| Vapor pressure at 20°C | 1.4 x 10-4 mm Hg |
| EPA toxicity classification | Class II |
| ACGIH TLV-TWA | 0.1 mg/m3 (skin) |
| NIOSH REL-TWA | 0.1 mg/m3 (skin) |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | 9 x 10-5 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | A4 |
| EPA | NA |
| IARC | NA |
|
NA = not available. |
|
Table 7.9
Formulations of Diazinon Available During ODS/DS
|
NSN |
|
|
Formulation (%) |
Unit Size | Application Directions |
| 6840-00-753-5038 | Diazinon Dust | Powder | 2 | 25-lb | Dust as provided |
| 6840-00-782-3925 | Diazinon 4E | Liquid | 48EC | Pail | Prepare solution |
Dichlorvos
General Information. Dichlorvos is used to control household and stored-product insects. It is effective against flies, aphids, spiders, and caterpillars, acting as both a contact and a stomach poison. Dichlorvos is also used as a fumigant and has been used to make pet collars and pest strips.
The chemical identity of dichlorvos is shown in Table 7.10, and Table 7.11 summarizes its physical and chemical properties.
Table 7.10
Chemical Identity of Dichlorvos
| Characteristic | Information |
| Chemical class | Organophosphate |
| Chemical name | 2,2-dichlorovinyl dimethyl phosphate |
| Trade names | SD 1750, Astrobot, Atgard, Canogard, DDVP, Dedevap, Dichlorman, Divipan, Equigard, Equigel, Estrosol, Herkol, No-Pest Strip, Nogos, Nuvan, Task, Vapona, Vaportape II, Verdisol |
| Chemical formula | C4H7Cl2O4P |
| CAS Registry number | 62-73-7 |
Table 7.11
Physical and Chemical Properties of Dichlorvos
| Property |
|
| Molecular weight | 220.98 |
| Color | Colorless to amber oily liquid |
| Odor | Mild chemical odor; aromatic odor |
| Water solubility at 20°C | 10 mg/mL |
| Partition coefficient (Kow) | 5.0 x 10-3 |
| Soil sorption coefficient (Koc) | 14.5 |
| Vapor pressure at 25°C | 1.2 x 10-2 mm Hg |
| EPA toxicity classification | Class I |
| ACGIH TLV-TWA | 0.9 mg/m3 (skin) |
| NIOSH REL-TWA | 1 mg/m3 (skin) |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | 100 mg/m3 |
| OSHA PEL-TWA | 1 mg/m3 (skin) |
| EPA IRIS RfD | 5 x 10-4 mg/kg/day |
| EPA IRIS RfC | 5 x 10-4 mg/m3 |
| Carcinogenicity classification | |
| ACGIH | A4 |
| EPA | B2 |
| IARC | 2B |
|
NA = not available. |
|
Availability and Recommended Use of Dichlorvos During ODS/DS. Dichlorvos was available during ODS/DS in the commercial product No-Pest Strip (Table 7.12).
Dichlorvos Residues. Few studies of dichlorvos residues from pest strips have been conducted in the past 25 years. However, trials have been carried out to determine the concentrations of dichlorvos that occur in the air of houses when strips are placed under conditions of normal domestic use. Ten trials were conducted in Australia and France between 1967 and 1970. Two trials were also carried out in the United Kingdom. Samples of air were taken at regular intervals throughout a three- to four-month period of each trial. The results from more than 3,000 samples of air showed that in the great majority of cases (97.2 percent), concentrations were 0.1 µg/L of air or less. Values ranged from less than 0.01 to 0.24 µg/L, the higher concentrations being associated with houses closed because of the absence of the householders or with several strips being in place in the house, or both. In each trial, the dichlorvos concentration in the air rose rapidly and then fell exponentially. In temperate-area trials, the concentration was at its highest one to two weeks after placing the strips, and the geometric mean of all the values at this time was 0.04 µ/L. Three months after placement, the mean concentration was 0.01 µg/L. The two U.K. trials resulted in the same dichlorvos concentrations in air and the same rate of decline of the concentrations.
Table 7.12
Formulations of Dichlorvos Available During ODS/DS
| NSN | Name | Form |
|
Unit Size | Application Directions |
| 6840-00-142-9438 | No-Pest Strip | Solid |
|
80 g/strip | Hang indoors; 900-1,200 ft3 /strip |
Ventilation apparently is the most important factor in determining the level of dichlorvos in the air of a room. The Australia trials were conducted in Brisbane, where houses are constructed to allow a flow of air and where doors and windows are open day and night. Concentrations of dichlorvos were low initially and quickly fell below the limit of determination. Increased ambient temperature increased the rate of emission of dichlorvos from the strip. However, in general, the increased ventilation associated with higher temperatures appeared to outweigh the increased rate of emission of insecticide, since concentrations in air tend to fall with increasing temperature. Some rooms, especially kitchens, are smaller than the volume recommended for placement of one strip. However, statistical analysis showed that initial concentrations in kitchens are no higher than in other rooms, and that the rate of decline of concentrations in kitchens is significantly higher than the rate in other rooms (Elgar, 1972).
Two trials assessed dichlorvos residues in food prepared in kitchens in which strips were placed. Samples, each of which consisted of the combined food and drink for an adult for one day, were taken at intervals during the trials. The food items were exposed and the meals prepared, including any cooking, in the way normal to the household. The residue concentrations from all the individual samples were less than 0.1 ppm. In one trial, the mean dichlorvos levels in samples taken seven, 42, and 70 days after the strips were hung were 0.03, 0.03, and 0.02 ppm, respectively. In the other trial, the mean concentrations were a little lower--0.02, 0.02, and less than 0.01 ppm at the same times. The items of food and the way they were processed varied widely from sample to sample and also between the two trials (conducted in the United Kingdom and France, respectively). However, the residue concentration in a sample did not appear to be correlated with the food items or with the manner of processing. No relationship was observed between the volume of the kitchens used, which varied from 14 m3 to 45 m3, and the level of residues found in the samples (Bosio, 1972).
Malathion
General Information. Malathion is a wide-spectrum insecticide that was introduced in 1950. It is used to control sucking and chewing insects on fruits and vegetables and also to control mosquitoes, flies, household insects, and animal parasites.
The chemical identity of Malathion is shown in Table 7.13, and Table 7.14 summarizes its physical and chemical properties.
Availability and Recommended Use of Malathion During ODS/DS. Malathion was primarily used as an outdoor spray during ODS/DS to control mosquitoes and flies. Table 7.15 lists the formulations that were available.
Environmental Characteristics of Malathion. Malathion displays little persistence in soil, with rapid degradation (Howard, 1991) and reported half-lives in the field ranging from one to 25 days (Wauchope et al., 1992). It does bind moderately to some soils and could contaminate groundwater or surface water in some cases. In air, malathion is rapidly broken down by sunlight, with a reported half-life of approximately one and one-half days (Howard, 1991).
Table 7.13
Chemical Identity of Malathion
| Characteristic | Information |
| Chemical class | Organophosphate |
| Chemical name | Diethyl (dimethoxyphosphinothioyl) thiobutanedioate |
| Trade names | Carbophos; Celthion; Cythion; Dielathion; El 4049; Emmaton; Exathios; Fyfanon; Hilthion; Karbofos; Malathion; Maltox |
| Chemical formula | C10H19O6PS2 |
| CAS Registry number | 121-75-5 |
Malathion Residues. Most studies of malathion residues have been conducted on edible products; however, a few studies have focused on malathion residues on skin and fabrics. Patches of fabrics exposed to pesticide spray formulations lost substantial quantities of the chemicals within four to six hours. Fabrics were cotton or 1:1 cotton-polyester blends, knitted or woven, unfinished or finished. Deposition and retention of pesticide-bearing particulates appeared to depend on mechanical restrictions related to fabric weave and on the electrokinetic potential of fabric surfaces (Serat, 1982).
Table 7.14
Physical and Chemical Properties of Malathion
| Property | Information |
| Molecular weight | 330.36 |
| Color/form | Clear, brown to colorless liquid |
| Odor | May be garlic-like |
| Water solubility at 25°C | 145 mg/L |
| Partition coefficient (Kow) | 560 |
| Soil sorption coefficient (Koc) | 1,800 |
| Vapor pressure at 30°C | 4 x 10-5 mm Hg |
| EPA toxicity classification | Class III |
| ACGIH TLV-TWA | 10 mg/m3 (skin) |
| NIOSH REL-TWA | 10 mg/m3 (skin) |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | 250 mg/m3 |
| OSHA PEL-TWA | 15 mg/m3 (total dust) (skin) |
| EPA IRIS RfD | 2 x 10-2 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | A4 |
| EPA | NA |
| IARC | 3 |
|
NA = not available. |
|
Table 7.15
Formulations of Malathion Available During ODS/DS
| NSN | Name | Form | Formulation (% by wt) | Unit Size | Application Method |
| 6840-00-685-5438 | E5 Malathion | Liquid |
|
5-gal can | Hand sprayer |
| 6840-00-655-9222 | Malathion EC | Liquid |
|
1-gal pail | Hand sprayer |
| 6840-00-926-1481 | Malathion ULV | Liquid |
|
55-gal drum | ULV sprayer |
| 6840-01-169-1842 | Malathion ULV | Liquid |
|
5-gal can | ULV sprayer |
In one residue study, malathion was sprayed using a truck-mounted ULV aerosol generator. Malathion concentrations were measured at selected positions on live, stationary human subjects wearing protective clothing and placed along a transect at right angles to the path of the truck. Two standing subjects were exposed downwind to the malathion spray at 7.6 m and 15.2 m. A third subject was exposed while jogging in the same direction as the spray vehicle and 1.5 m from the spray path. No significant differences (p < 0.05) in total amount of malathion deposited on subjects were demonstrated. The average amounts of malathion deposited at ground level at 15.2, 30.4, and 91.2 m were not significantly different (p > 0.05). Malathion dermal residues were compared with the acute LD50 value (4,100 mg/kg) for a 70-kg adult male. Calculated malathion dermal exposures were less than the acute lethal dose for a human subject by four orders of magnitude or more (Moore, 1993).
Carbamates were originally extracted from the calabar bean, which grows in West Africa. The extracts of this bean contain physostigmine, a methylcarbamate ester (Baron, 1991). Carbamates are derivatives of carbamic acid, as OPs are derivatives of phosphoric acid. Like the OPs, carbamates as a class are not generally persistent in the environment.
The use of carbamates as insecticides began in the 1950s, and approximately 25 carbamate compounds are in use today as pesticides or pharmaceuticals. Carbamates are among the most popular pesticides for home use, both indoors and on gardens and lawns. Although not identified by OSAGWI as a pesticide of concern for Gulf War illnesses, carbaryl is perhaps the best known and most applied carbamate pesticide, used primarily for lawns and gardens. Pyridostimine bromide (PB) is also a carbamate, though not a pesticide. PB tablets were taken as a prophylactic treatment for nerve agents during ODS/DS, and a literature review of PB as it pertains to Gulf War illnesses was published as a separate volume in this series (Golomb, 1999).
Bendiocarb
General Information. Bendiocarb is a broad-spectrum insecticide used to control disease vectors such as mosquitoes and flies, as well as household and agricultural pests. Most formulations of bendiocarb are registered for general use, except for Turcam and Turcam 2.5G, which are restricted products. Perhaps the best known bendiocarb product is Ficam. Formulations include dusts, granules, ULV sprays, and wettable powders.
The chemical identity of bendiocarb is shown in Table 7.16, and Table 7.17 summarizes its physical and chemical properties.
Availability and Recommended Use of Bendiocarb During ODS/DS. Bendiocarb was primarily available during ODS/DS as a wettable powder for indoor surface treatment. According to OSAGWI, the specific product available was a 76 percent solid known as Ficam, NSN 6840-01-087-6672. Ficam can be applied indoors to control fleas, ticks, cockroaches, and stored-product pests at concentrations of 0.25 to 0.5 percent in water, and a 1 percent solution in water can be applied indoors to control mosquitoes.
Table 7.16
Chemical Identity of Bendiocarb
| Characteristic | Information |
| Chemical class | Carbamate |
| Chemical name | 2,2,dimethyl-1,3-benzodioxol-4-yl methylcarbamate; 2,3-isopropylidenedioxyphenyl methylcarbamate |
| Trade names | Dycarb, Ficam, Garvox, Multamat, Multimet, Niomil, Rotate, Seedox, Tattoo, Turcam |
| Chemical formula | C11H13NO4 |
| CAS Registry number | 22781-23-3 |
Table 7.17
Physical and Chemical Properties of Bendiocarb
| Property | Information |
| Molecular weight | 223.23 |
| Color/form | White solid |
| Odor | Odorless |
| Water solubility at 20°C | 40 mg/L |
| Partition coefficient (Kow) | 50 |
| Soil sorption coefficient (Koc) | 570 |
| Vapor pressure at 25°C | 5 x 10-6 mm Hg |
| EPA toxicity classification | Class II |
| ACGIH TLV-TWA | NA |
| NIOSH REL-TWA | NA |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | 1.3 x 10-3 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | NA |
| EPA | NA |
| IARC | NA |
|
NA = not available. |
|
Environmental Characteristics of Bendiocarb. Bendiocarb has low soil persistence, with half-lives in soil of from one to four weeks, depending on soil type (Worthing, 1987; Kidd and James, 1991), and may display slight mobility in some soils (Swann et al., 1983). Bendiocarb does not volatilize significantly from soil surfaces (Wright et al., 1981). Bendiocarb is degraded in solution by hydrolysis and does not accumulate in water (Kamrin, 1997).
Bendiocarb Residues. In one study, a 1 percent formulation of bendiocarb was applied in a furnished office. Airborne concentrations peaked during application at 2.7 �g/m3; after two hours, the level decreased to 0.7 at 2.7 �g/m3, and the levels after one and two days were 0.17 and 0.14 at 2.7 �g/m3, respectively. Bendiocarb was deposited during application on aluminum plates (detected at concentrations of 2.1 to 3.1 ng/cm2) and detected in floor and furniture wipe samples at concentrations of 11 to 25 ng/cm2 (Currie et al., 1990). In another study, a 0.5 percent wettable powder suspension of bendiocarb was applied to 49 dormitory rooms. Bendiocarb was detected in air samples at 7.7 �g/m3 on the day of application and 1.3 �g/m3 after one day; it was not detected on the two subsequent days (Wright et al., 1981).
Methomyl
General Information. Methomyl is classified by the EPA as highly toxic to humans and is a restricted-use pesticide[1] due to its high acute toxicity to humans. It was introduced in 1966 as a broad-spectrum insecticide and was first registered in 1968. It was re-registered in 1998, with the EPA concluding that methomyl products will not pose unreasonable risk to humans or the environment when labeled and used correctly (USEPA, 1998b). Methomyl is effective both as a contact and a systemic insecticide. That is, methomyl can kill insects upon direct contact and also after absorption, especially after the insect feeds on a treated plant.
There are currently 15 methomyl products registered for a variety of uses, including agricultural use and fly control in livestock quarters, refuse containers, and commercial premises. There are no registered homeowner uses of methomyl. Methomyl can be formulated as a wettable powder, a soluble concentrate or liquid, a dust, or a solid bait.
The chemical identity of methomyl is shown in Table 7.18, and Table 7.19 summarizes its physical and chemical properties.
Availability and Recommended Use of Methomyl During ODS/DS. Methomyl was used exclusively as a fly bait during ODS/DS. According to OSAGWI, the specific product available was a 1 percent methomyl formulation known as Flytec, NSN 6840-01-183-7244. This pellet bait is packaged in five-pound cans and is intended to be used outdoors at a rate of 0.5 lb/1,000 ft2.
Table 7.18
Chemical Identity of Methomyl
| Characteristic | Information |
| Chemical class | Carbamate |
| Chemical name | S-methyl N-[(methylcarbamoyl)oxy] thioacetimidate |
| Trade names | Acinate, Agrinate, DuPont 1179, Flytek, Kipsin, Lannate, Lanox, Memilene, Methavin, Methomex, Nudrin, NuBait, Pillarmate, SD 14999 |
| Chemical formula | C5H10N2O2S |
| CAS Registry number | 16752-77-5 |
Table 7.19
Physical and Chemical Properties of Methomyl
| Property | Information |
| Molecular weight | 162.2 |
| Color/form | Colorless to white crystalline solid |
| Odor | Slight sulfurous |
| Water solubility at 25°C | 10 g/L |
| Partition coefficient (Kow) | 3.98 |
| Soil sorption coefficient (Koc) | 51, 72, 160 (depending on reference) |
| Vapor pressure at 25°C | 5.0 x 10-5 mm Hg |
| EPA toxicity classification | Class I |
| ACGIH TLV-TWA | 2.5 mg/m3 |
| NIOSH REL-TWA | 2.5 mg/m3 |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | 2.5 x 10-2 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | A4 |
| EPA | E |
| IARC | NA |
|
NA = not available. |
|
Propoxur
General Information. Propoxur was introduced in 1959 as an insecticide, and it was first registered in the United States in 1963. Like methomyl, it has both contact and systemic activity against insects and is used on a variety of pests in both agricultural and non-agricultural applications. Propoxur is a general-use pesticide, although some formulations may be for professional use only.
Propoxur is characterized by a fast knockdown and a long residual effect, which makes it a popular choice for pest control. It is used primarily indoors. Propoxur is available in a variety of formulations, including emulsifiable concentrate, wettable powder, dustable powder, granules, aerosol generator, smoke generator, and baits.
Generally, propoxur is moderately toxic to mammals when ingested and slightly toxic in inhalation and dermal exposures. Mild cases of poisoning were noted during WHO-sponsored, wide-scale spraying of propoxur for malaria control. Applicators who used propoxur on a regular basis showed a pronounced daily fall in whole blood cholinesterase activity and a distinct recovery after exposure ceased. No adverse cumulative effects were demonstrated (ACGIH, 1986, p. 499).
The chemical identity of propoxur is shown in Table 7.20, and Table 7.21 summarizes its physical and chemical properties.
Availability and Recommended Use of Propoxur During ODS/DS. During ODS/DS, propoxur (Baygon) was used indoors as a crack and crevice treatment to control pests such as cockroaches; it was also sprayed on building surfaces and screens to control outdoor pests (Table 7.22).
Environmental Characteristics of Propoxur. Propoxur is generally not known to be strongly absorbed by soil and is readily degraded in water (it has a half-life of from one day to one week). Propoxur biodegradation in soil and water is rapid, particularly at high temperatures. On the basis of propoxur’s vapor pressure and water solubility, its volatilization from water is considered negligible. It hydrolyzes in water at a rate of 1.5 percent per day in a 1 percent aqueous solution at pH 7.0 (USEPA, 1988b). Propoxur degrades rapidly at more alkaline pH values.
Table 7.20
Chemical Identity of Propoxur
| Characteristic | Information |
| Chemical class | Carbamate |
| Chemical name | 2-(1-methylethoxy)phenyl methylcarbamate; o-isopropoxyphenyl N-methylcarbamate |
| Trade names | Aprocarb, Bay 39007, Bay 9010, Baygon, Bayer 39007, Bifex, Blattanex, Brifur, Bolfo, BO Q 5812315, ENT 25671, Invisi-Gard, OMS 33, PHC, Pillargon, Prentox Carbamate, Propogon, Propyon, Rhoden, Suncide, Sendran, Tendex, Unden, Undene |
| Chemical formula | C11H15NO3 |
| CAS Registry number | 114-26-1 |
The field half-life of propoxur has been reported to be 14 to 50 days. It has a low affinity for soil binding and may therefore be mobile in many soils (Wauchope et al., 1992). A USEPA study found virtually no loss of propoxur from silty loam soil six months after application, but 25 percent of applied propoxur (Baygon) was lost from sand after 100 days (USEPA, 1988). Other studies have shown that propoxur is mobile in soils with more organic content as well (e.g., silty clay, silty loam, and sandy loam) (Kamrin 1997).
Table 7.21
Physical and Chemical Properties of Propoxur
| Property | Information |
| Molecular weight | 209.24 |
| Color/form | White to cream or tan crystalline solid |
| Odor | Odorless to faint characteristic |
| Water solubility at 25°C | 1,750 mg/L |
| Partition coefficient (Kow) | 1.4 |
| Soil sorption coefficient (Koc) | 30 |
| Vapor pressure at 20°C | 3 x 10-6 mm Hg |
| EPA toxicity classification | Class II for oral exposures; Class III for dermal and inhalation exposures |
| ACGIH TLV-TWA | 0.5 mg/m3 |
| NIOSH REL-TWA | 0.5 mg/m3 |
| NIOSH REL-STEL | NA |
| NIOSH IDLH value | NA |
| OSHA PEL-TWA | NA |
| EPA IRIS RfD | 4 x 10-3 mg/kg/day |
| EPA IRIS RfC | NA |
| Carcinogenicity classification | |
| ACGIH | A3 |
| EPA | NA |
| IARC | NA |
|
NA = not available. |
|
Table 7.22
Formulations of Propoxur Available During ODS/DS
| NSN | Name | Form | Formulation (%); Application | Unit Size | Application Method |
| 6840-01-033-2623 | Baygon | Wettable powder | 70; suspension of 2 oz/gal to treat buildings and screens |
5-lb bag | Hand spray gun |
| 6840-00-180-6069 | Baygon | Oil solution | 1; crack and crevice |
1-gal can | Hand spray gun |
| 6840-01-027-3865 | Baygon | Liquid | 14.7; crack and crevice treatment |
1-gal can | Hand spray gun or power sprayer |
|
Source: Provided by OSAGWI. |
|||||
Propoxur Residues. The EPA has estimated a cancer risk of 4.5 x 10-7 for applicators of crack and crevice treatments (USEPA, 1997). (Residential cancer risks of less than 1 x 10-6 are considered not to be of concern.) It should be noted that this estimation is based on a suite of assumptions regarding propoxur application and exposure to target individuals. While these assumptions were made conservatively, they should be reviewed if unique information regarding exposures during the Gulf War is discovered.
Following crack and crevice treatment, individuals can be exposed to propoxur via inhalation or dermal contact with residue. In its approval of residue studies following crack and crevice treatments, the EPA pooled concentration data to yield an average air concentration of 5.1 µg propoxur/m3 over a one-year period which included a 64-ounce treatment of a 1.1 percent propoxur solution by weight (total of 0.73 ounce) for annual cleanout treatment as well as 11 treatments of 16 ounces of a 0.5 percent propoxur solution by weight (a total of 0.083 ounce per treatment) (USEPA, 1997). In another study, the airborne concentration of propoxur after application as a 1.1 percent emulsion to a 61.2-m3 dormitory room whose approximate temperature and humidity were 25°C and 60 percent was 15.4 µg propoxur/m3. After one, two, and three days, the levels declined to 2.7, 1.8, and 0.7 µg propoxur/m3, respectively (Wright et al., 1981). Propoxur was also detected in the indoor air of seven of nine households in a pilot project of pesticide exposure. The concentrations in areas of high household activity (e.g., kitchens) ranged from none detected to 0.0039 µg/m3, with a mean of 0.042 µg/m3 (Lewis et al., 1988).
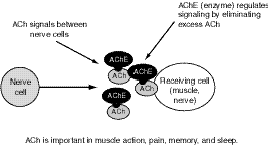
OP (like carbamate) agents act by binding to, and inhibiting the normal action of, acetylcholinesterase (AChE), an enzyme. Acetylcholine (ACh) is a major neurotransmitter, or nerve-signaling chemical, that acts as a signaling chemical both in the brain and elsewhere in the body; for example, it is the main signaling chemical used by nerves to tell muscles to contract. AChE breaks down (metabolizes) ACh in the synapse, the area where a nerve sends signals to another nerve or to a muscle (see Figure 7.1). When AChE is inhibited by an OP, an excessive accumulation of ACh occurs in the synapse, followed by excessive binding of ACh to the receptors on the receiving cell (see Figure 7.2). Consequently, cells are excessively stimulated. The increase in ACh action leads to symptoms characteristic of increased ACh activity at peripheral and, to varying degrees, central ACh receptors, which fall largely into two classes, nicotinic and muscarinic.

Figure 7.1--Normal Nerve Signals
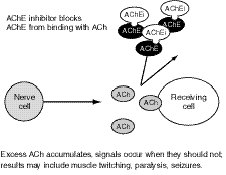
Figure 7.2--Effect of AChE Inhibitors Such as OPs
Nicotinic effects in the periphery, consisting of effects at the neuromuscular junction as well as at other nicotinic sites, include fasciculations and muscle contractions, muscle pain, generalized weakness and fatigue, tachycardia, hypertension, hyperglycemia, pallor, paresthesias, and, rarely, mydriasis (Minton and Murray, 1988; Leveridge, 1998).
Muscarinic effects in the periphery include secretions from glands and contraction of smooth muscles, leading to such symptoms and signs as lacrimation (secretions from tear ducts), hypersalivation, diaphoresis (sweating), rhinorrhea (runny nose), bronchorrhea (bronchial secretions), bronchial constriction, cyanosis, nausea, vomiting, abdominal cramps and diarrhea (from increased peristalsis and increased intestinal secretions), urinary urgency or incontinence (from contractions of sphincteric muscles), miosis, blurred vision, bradycardia, heart block, hypotension, dyspnea (shortness of breath), and pulmonary edema (Minton and Murray, 1988; Leveridge, 1998).
Central muscarinic and nicotinic effects include insomnia and sleep abnormalities, headaches, dizziness, effects on mood (depression, anxiety), effects on personality (aggressiveness, irritability, and paranoia), effects on cognition (confusion, enhancements and reductions in measures of attention, concentration, memory, learning, and psychomotor speed), tremor, ataxia, dysarthria, hypotension, respiratory depression or arrest, convulsions, and coma (Devinsky et al., 1992; Minton and Murray, 1988; Leveridge, 1998). In addition, AChE inhibitors may affect thermoregulation and response to stress.
Acute Effects
Symptoms that occur acutely with OP (and carbamate) toxicity can span a range from mild tremors to more severe muscle contractions, impaired cognition, dizziness, shortness of breath, and vomiting. In severe cases, respiratory failure and death can result. The severity of symptoms is related to the amount and route of exposure. The literature contains no systematic reports of acute toxicity resulting from any pesticide exposures during ODS/DS; however, it is conceivable that mild symptoms related to such exposures could have occurred and medical care was not sought, or the symptoms may have been attributed to other factors. For this reason, this report focuses primarily on chronic exposures and long-term effects.
Acute toxicity for both OP and carbamate poisoning may be complicated by ventricular arrhythmias, CNS depression, seizures, or respiratory failure; and relapse may occur after seemingly successful treatment (Bardin et al., 1994). Additional problems with acute toxicity that have been described less frequently include renal failure, which may be associated with proteinuria (Wedin et al., 1984; Albright et al., 1983), and pancreatitis, which has been reported to occur with exposure to AChE-inhibiting pesticides--most commonly, OPs--in adults and children and may be painless and go undetected. Hyperamylasemia is particularly common (Weizman and Sofer, 1992; Dagli and Shaikh, 1966; Dagli et al., 1981; Dressel et al., 1979; Lankisch et al., 1990; Lee et al., 1997; Marsh et al., 1988; Moore and James, 1981).
Most of what is known about symptoms associated with acute exposures to pesticides, including OPs, comes from studies of patients who were involved in accidental exposures or mishandling/misapplication of pesticides. For example, Saadeh et al. (1996) evaluated clinical manifestations of 70 adult patients (33 males, 37 females) in North Jordan who were admitted to a teaching hospital for acute carbamate or OP poisoning associated with accidents, suicide attempts, or occupational exposures. Clinical manifestations reported are listed in Table 7.23.
Table 7.23
Clinical Manifestations Following Acute Carbamate or OP Poisoning
(n=70)
|
Clinical Effect |
|
| Miosis |
|
| Nausea and vomiting |
|
| Excessive salivation and bronchial secretions |
|
| Headache and dizziness |
|
| Fever |
|
| Abdominal pain or cramps |
|
| Muscular twitching and fasciculation |
|
| Pulmonary edema |
|
| Sinus tachycardia |
|
| Coma |
|
| Sinus bradycardia |
|
| Diarrhea or urinary incontinence |
|
| Hypertension |
|
| Blurred vision |
|
| Hypotension |
|
| Fits |
|
| Tremors |
|
| Hallucination |
|
| Respiratory muscle paralysis |
|
| Source: Saadeh et al. (1996). | |
OPIDN and Intermediate Syndrome. The specific cases of OP-induced delayed neuropathy (OPIDN)--also called OP-induced delayed polyneuropathy (OPIDP)--and intermediate syndrome will be discussed only briefly. Although each is a well-described, somewhat delayed phenomenon, both are generally considered to require significant acute exposure, which would be expected to have occurred very seldom during ODS/DS. Moreover, the symptoms have only passing relevance to those being reported by ill PGWV. However, they do provide an example of delayed, and in one instance long-lasting, illness whose onset is precipitated by, but not tied to drug levels of, AChE-inhibiting agents.
OPIDN is a form of delayed clinical and pathological response to OPs. There is a chronic central and peripheral distal axonopathy affecting both sensory and motor fibers, with a secondary myelinopathy leading to clinical symptoms consisting of a progressive phase (primarily a peripheral neuropathy) followed by a stationary phase and an improvement phase. Longer-diameter motor and sensory fibers may or may not be more susceptible (Jamal, 1995). In the progressive phase, there is a distal symmetric sensory and motor neuropathy that affects primarily the lower limbs. Initial pain, burning, and tingling sensations of the lower extremities may later lead to hypoesthesia in a stocking or stocking-and-glove distribution. Weakness of the legs may spread to the hands, and foot drop, steppage gait, ataxia, a positive Romberg sign, and even possibly paraplegia or quadriplegia (bilateral flaccid paralysis) may occur in severe cases. In the stationary phase, lasting from three to 12 months after symptom onset, the sensory symptoms may disappear, but weakness persists. In the improvement phase, from six to 24 months after onset of neurological deficits, improvement occurs in reverse order to symptom onset. Typically, onset follows significant acute OP toxicity. There is some question about whether more-subtle cases of OPIDN may be more common than previously thought (Jamal, 1995; Schaumburg and Berger, 1993).
Symptoms typically follow exposure to selected OPs by one to three weeks (Abou-Donia, 1981; Abou-Donia and Lapadula, 1990; Jamal, 1995). Apparently complete recovery may occur in mild cases. The likelihood of recovery has been estimated at less than 2 percent in severe cases (Geoffroy et al., 1960; Minton and Murray, 1988); hands may show great improvement, but paralysis below the knees may remain. Later stages of neurological deficit involve central rather than peripheral lesions in the spinal cord. These lesions are unmasked as peripheral neuropathy abates and are characterized by spasticity (increased muscle tone) and exaggerated knee jerk (Abou-Donia and Lapadula, 1990). Chronic neuropsychological effects have also been reported to occur (Lotti, 1992; Karczmar, 1984).
Induction of OPIDN is thought to be related not to AChE inhibition, but to phosphorylation of the enzyme neurotoxic esterase, or neuropathy target esterase (NTE), a membrane bound carboxylesterase, followed by "aging" of the OP-NTE complex. While OPs generally inhibit AChE, only a selected subset also inhibit lymphocyte and brain NTE, and inhibition of NTE has been useful in predicting which agents can produce OPIDN (Barrett and Oehme, 1985; Gordon et al., 1983; Lotti and Johnson, 1978; Lotti and Moretto, 1986). It has been thought that induction of OPIDN requires at least 70 percent inhibition of NTE. Not all OPs that inhibit NTE produce OPIDN, and, like carbamates, those that do not typically produce it may protect against it if administered before a neurotoxic OP exposure (Pope et al., 1993; Pope and Padilla, 1990). Not all animals are similarly susceptible; for example, cats, primates, and hens develop OPIDN, while rats are relatively refractory. "Non-neuropathic" OPs and carbamates may potentiate OPIDN if administered after the neuropathic OP, or may even produce it in animals who would not have been susceptible from the neuropathic OP exposure alone (Pope et al., 1993; Pope and Padilla, 1990). Moreover, as noted in the section on carbamates, delayed neuropathy has been reported following carbamate exposure without known OP exposure.
Intermediate syndrome is a delayed neurotoxic condition (also called Type II paralysis) that has only recently been defined. It occurs after the acute OP cholinergic crisis, but before the development of OPIDN--typically one to four days after acute poisoning (Senanayake and Karalliedde, 1987). The main symptoms are a proximal neuropathy and muscle weakness (e.g., in the neck flexors, motor cranial nerves, proximal muscles of the limbs, and respiratory muscles), with recovery usually within several weeks (Jamal, 1995; Leon et al., 1996; Mani et al., 1992). Respiratory insufficiency or ventilatory failure may result from paralysis of the respiratory muscles, and artificial ventilation may be required. Deep tendon reflexes may be absent. A number of OPs have been implicated, including fenthion, methyl-parathion, parathion, and dimethoate; and while the syndrome may occur more commonly with certain compounds--perhaps those with higher lipid solubility--it is not confined to a few distinct compounds (De Bleecker, 1993). Recovery usually occurs at between four and 18 days. The necrotizing myopathy seen with AChE inhibitors has been suggested as a cause (Senanayake and Karielledde, 1987), as has persistent AChE inhibition (De Bleecker, 1993)
Other Persistent Effects Following Acute Exposure. In some cases, acute exposure to OPs has been associated with effects manifesting from days to years later. For example, Markowitz et al. (1986) compared 22 seamen accidentally exposed to a cloud of malathion from a nearby overheated tank with 21 seamen controls from a distant tanker. The subjects were interviewed 12 days after exposure for symptoms, using a medical review of body symptoms and a "demoralization" scale reflecting psychological symptoms of distress. For 17 of 18 symptom classes, the OP-exposed seamen reported symptoms more frequently than did the control seamen by at least a factor of two (p < 0.001, sign test). The symptoms are listed in Table 7.24 in descending order of frequency of occurrence.
Thrasher et al. (1993) reported persistent symptoms of fatigue, headache, joint and muscle pain, memory problems, upper and lower respiratory problems, GI disturbance, dizziness, atopy, and antibiotic sensitivity from one to four-and-one-half years after reported chlorpyrifos exposure in 12 subjects.
Twenty-nine lettuce harvesters who were exposed to the OP mevinphos and presented to the emergency room for acute cholinergic symptoms were followed for 12 weeks (Coye et al., 1986). Initial symptoms of headache, eye irritation, blurred vision, and pruritus persisted at least 10 weeks in some of the harvesters. Similarly, Tabershaw and Cooper (1966) followed up on 114 of 232 patients who had experienced acute OP toxicity three years earlier. The duration and nature of the OP exposures were not defined. Nine percent reported persistent headaches and anxiety three years later, and many cited blurred vision. The vision problems were attributed by the authors to presbyopia, but no control rates were given to support this attribution. Few long-term sequelae were reported. Symptoms lasting longer than six months were reported by 38 percent of the subjects.
Table 7.24
Symptom Rates in Malathion-Exposed Subjects
| Symptom Class |
|
|
| Head (headache, dizziness) |
|
|
| GI |
|
|
| Nose or throat |
|
|
| Sleep problems |
|
|
| Abdominal (pain, nausea) |
|
|
| Energy |
|
|
| Eyes/vision |
|
|
| Chest or respiratory (chest pain, shortness of breath, cough) |
|
|
| Mouth/lips/teeth |
|
|
| Muscle/joint pain or neuropathy |
|
|
| Appetite |
|
|
| Urinary |
|
|
| Skin/hair |
|
|
| Motor function |
|
|
| Heart rhythm (pound, skip) |
|
|
| Temperature (fever/chills) [a] |
|
|
| Genitals or sexual function |
|
|
|
Source: Markowitz et al. (1986).
*p < 0.05. **p < 0.01. [a] The context of fever and chills is extremely important in interpreting this finding. Fever and/or chills during infectious disease is arguably not disruption of thermoregulation, but rather a normal response. Increased fever and chills could, therefore, signal diminished resistance to infectious disease—not altered thermoregulation. On the other hand, unexplained fevers, etc., might signal altered thermoregulation. Malathion-exposed seamen, evaluated days after exposure, experienced increased fever and chills, and there was no direct evidence to suggest that infection was present. Increased fever and chills could also result from susceptibility to occult, undiagnosed infection. Changes in immune function could be theorized to lead to such increased susceptibility, perhaps selectively to certain viral, parasitic, and intracellular bacterial infections that may relate to changes in T-helper cell cytokine profiles. |
||
Thirty-six male Nicaraguan agricultural workers 15 to 44 years of age at the time of hospitalization for OP intoxication were compared with male controls (a close male friend or sibling never treated for pesticide poisoning and matched by age, within five years, to each exposed participant) approximately two years following the acute toxicity episode (Rosenstock et al., 1991). Ongoing lower-level OP exposure was not excluded, and the duration of exposure was not reported. Symptoms related to CNS function were assessed by a validated test of self-reported difficulties in memory and concentration, along with headache, fatigue, depression, and irritability (Scandinavian questionnaire, p < 0.01) (Hogstedt et al., 1984). It was observed that "the exposed group did much worse than the control group on all neuropsychological subtests." Findings were adjusted for vocabulary score, considered moderately resistant to cortical insult. Differences in neuropsychological performance could not be explained by other factors examined.
Midtling et al. (1985) studied cauliflower workers who experienced acute poisoning by OP insecticides mevinphos (Phosdrin) and phosphamidon (Dimecron). The workers had begun work tying leaves over the heads of the plants only six hours after the field had been sprayed. Sixteen such workers were followed in weekly clinics with interviews and plasma and red blood cell (RBC) cholinesterase levels. Comparatively non-persistent symptoms (i.e., they had typically resolved by 10 weeks) included nausea, dizziness, vomiting, abdominal pain, ataxia, and night sweats or insomnia. Symptoms that persisted in at least three of the 16 subjects at 10 weeks or more included blurred vision/vision disturbance (56 percent), headache (25 percent), anxiety (41 percent), weakness, and anorexia. Symptoms persisted for up to 10 weeks, varying by symptom and individual. Six of the subjects initially had RBC AChE values within the normal laboratory range, but follow-up testing showed activity to have been significantly inhibited.
Chronic Effects
Chronic health effects are of greater relevance to Gulf War illnesses than are acute effects. In addition, most reports of acute effects center on large exposures with severe acute debility, which is not known to have been reported following pesticide exposure during ODS/DS. As with other pesticides, most of what is known about the effects of persistent OP exposure in humans is based on observational studies. These studies are usually focused on occupational exposures, and they usually involve a mixture of pesticides and possibly other compounds. They often assess the symptoms of a study group that is exposed to pesticides seasonally. Further, there is often a combination of acute and chronic exposures and effects, and this combination is frequently undefined. For example, a seasonal agricultural worker who mixes and sprays pesticides is almost certainly exposed chronically but also risks acute exposures to higher concentrations because of accidents during mixing, loading, and handling.
For example, in a study of fenthion (OP) sprayers presumed to be exposed chronically and seasonally, serum AChE was found to be significantly inhibited in the exposed group (p < 0.01), making it difficult or impossible to distinguish the effects of acute inhibition from chronic effects (Misra et al., 1994). In that study, 32 sprayers with a mean age of 32.1 years (range = 19 to 55 years) and a mean exposure of 10.5 years (range = one to 14 years) were compared with 25 non-exposed hospital employees matched for age (mean 30.0, range = 18 to 50 years), sex, educational status, and socioeconomic status. Significant deficits were observed in the following neuropsychological evaluations of the sprayer group:
In a study that compared 57 OP-exposed male fruit-tree farmers with 42 male controls, Fiedler et al (1997) found slower reaction times among the OP-exposed farmers. This group had significantly slower reaction times with the dominant hand than did the control group, which included hardware-store owners and cranberry/blueberry growers thought to be unexposed to OPs. The fruit-tree farmers were exposed seasonally to OPs and were "without evidence of an acute poisoning episode." Symptoms were not assessed, and the recency of last exposure was not provided, so recent OP exposures could not be excluded.
In another examination of 300 subjects exposed for years to mixed pesticides, including OPs, the longest exposures (>20 years) were associated with higher frequencies of weeping (p < 0.05) and irritability (p < 0.001) than were found in 300 control subjects (Amr et al., 1993). No additional values were reported for other symptoms in the long-term-exposure cases. AChE inhibition was widely present in the exposed subjects, indicating that many were exposed to OPs and/or carbamates, but information on other exposures was not provided.
Stephens et al. (1995) compared 145 male sheep farmers in the United Kingdom who were exposed to OPs used as sheep dips with 143 quarry workers who were presumed to be non-exposed. Licensed OP sheep dips in the United Kingdom contain either diazinon, a mixture of diazinon and chlorfenvinphos, or propetamphos as the active ingredient. Episodes of acute toxicity were neither required nor excluded in study participants, whose ages ranged from 16 to 65 years, and symptoms were not reported. While scores on the subjective memory questionnaire (evaluating perception of memory function) did not differ between the groups (p = 0.39), those who reported greater pesticide exposure history also displayed greater decrement in syntactic reasoning performance (F = 5.54, p < 0.0001). Further, for the general health questionnaire, reporting of at least five symptoms, regarded as indicating "vulnerability to psychiatric disorder," was more common among sheep dippers than among controls (OR = 1.5, 95 percent CI = 1.31-1.69, p = 0.035).
In another observational study, 146 U.K. sheep farmers were compared with 153 non-exposed quarry workers; the 10 most symptomatic and 10 least symptomatic farmers were compared with each other immediately after dipping and with 10 of the quarry workers (the selection process was not described) several months later on a standardized neurological exam (Beach et al., 1996). Some significant differences were observed between the two farmer groups. The symptomatic group had significantly smaller mean calf circumference (p = 0.033), an increased two-point discrimination distance in the hand (22 mm vs. 13 mm, p = 0.011), and an increased two-point discrimination distance in the foot (34 mm vs. 10 mm; p < 0.001). There was no significant difference in OP exposure between the two groups of sheep farmers. The authors suggest that "some neurological changes, albeit relatively subtle, had occurred as a consequence of long-term exposure to OP sheep dip at concentrations which had never induced sufficient symptoms that medical attention was sought." More specifically, the data suggest that this may occur selectively in a susceptible subset that may be identified by more pronounced acute changes on AChE-inhibitor exposure.
A prospective cohort study of Indonesian farmers and professional sprayers exposed to OPs resulted in a comprehensive list of symptoms putatively associated with seasonal OP exposure (see Table 7.25) (Kishi et al., 1995). Subjects were examined during the spraying season (n = 904) and the off-season (n = 1,392). (The different sample sizes imply that the samples were not identical in each test.) A trend was seen between neurobehavioral signs and symptoms and the use of multiple OP and carbamate pesticides. Tests of trends were positive for the factors shown in Table 7.26 (i.e., the factors were significantly linked to symptom reporting).
Table 7.25
Symptom Rates and Relative On-Season and Off-Season Risks
for Indonesian Farmers Engaged in OP Pesticide Spraying
|
Symptom Class |
|
(n = 1,392) |
Relative Risk |
95% CI |
| Neurobehavioral | ||||
| Fatigue | 60.2 | 20.4 | 3.0 |
|
| Dizziness | 20.8 | 4.1 | 5.1 |
|
| Insomnia | 16.8 | 2.4 | 7.1 |
|
| Blurred vision | 15.5 | 4.2 | 3.7 |
|
| Flushed face | 13.9 | 0.3 | 48 |
|
| Headache | 13.2 | 4.9 | 2.7 |
|
| Salivation | 13.1 | 0.8 | 17 |
|
| Excess sweating | 3.7 | 0.5 | 7.3 |
|
| Pallor | 2.9 | 0.7 | 4.2 |
|
| Hand tremor | 2.0 | 0.2 | 9.2 |
|
| Twitching eyelids | 1.5 | 0.3 | 5.4 |
|
| Staggering | 0.9 | 0.1 | 12 |
|
| Irritability | 0.3 | 0.9 | 0.3 |
|
| Loss of consciousness | 0 | 0 | NA |
|
| Intestinal | ||||
| Nausea | 10.8 | 1.7 | 6.6 |
|
| Queasiness | 5.4 | 1.1 | 5.0 |
|
| Belly pain | 3.1 | 1.6 | 1.9 |
|
| Constipation | 1.9 | 0.5 | 3.8 |
|
| Vomiting | 0.7 | 0 |
|
|
| Diarrhea | 0.3 | 0.5 | 0.6 |
|
| Respiratory | ||||
| Dry throat | 29.9 | 0.8 | 38 |
|
| Difficulty breathing | 18.5 | 2.0 | 9.2 |
|
| Chest pain | 13.6 | 2.7 | 5.1 |
|
| Sore throat | 5.2 | 1.1 | 4.8 |
|
| Cough | 4.4 | 6.2 | 0.7 |
|
| Runny nose | 1.9 | 4.4 | 0.4 |
|
| Epithelial/mucosal surfaces | ||||
| Stinging eyes | 15.2 | 0.8 | 19 |
|
| Itchy skin | 0.3 | 4.6 | 2.0 |
|
| Red eyes | 7.3 | 2.2 | 3.3 |
|
| Burning nose | 6.5 | 0 |
|
|
| White rash and scaling | 5.4 | 0.9 | 6.3 |
|
| Burning eyes | 5.1 | 0.1 | 35 |
|
| Itchy eyes | 3.7 | 0.1 | 25 |
|
| Blisters | 1.5 | 0.3 | 5.0 |
|
| Red skin | 0.8 | 0.3 | 2.6 |
|
| Eye discharge | 0.7 | 0.9 | 0.8 |
|
| Burning tongue | 0.6 | 0.1 | 6.0 |
|
| Abraded skin | 0.3 | 0 |
|
|
| Muscle | ||||
| Muscle stiffness | 54.0 | 18.6 | 2.9 |
|
| Muscle weakness | 22.8 | 13.5 | 1.7 |
|
| Muscle cramps | 1.8 | 0.7 | 2.6 |
|
|
NA = not available; NS = not significant. |
||||
Table 7.26
Factors Significantly Linked to Symptom Reporting in Indonesian Farmers Engaged in OP Spraying
| Factor |
|
| Sprayed since previous week | 0.00000 |
| Wore clothes unwashed since previous spray | 0.00000 |
| Used bottle to mix pesticide "cocktail" | 0.00000 |
| Feet wetted when pouring solution | 0.00000 |
| Body wetted by solution | 0.00000 |
| Shirt soaked with solution | 0.00000 |
| WHO hazard grade IB/II (% of pesticides) [a] | 0.0039 |
| Multiple use of hazardous pesticides [b] | 0.0001 |
|
[a] Proportion of pesticides used that are classified as highly hazardous or moderately hazardous. [b] Two or more grade IB/II pesticides (OP and carbamates) mixed together. |
|
While some study designs compare exposed and non-exposed populations, others employ a longitudinal study design of pre- and post-season observations. In one such study in Israel, neurobehavioral tests were administered to 90 subjects (51 occupationally exposed, 39 non-exposed) before and after the pesticide spraying season (Richter et al., 1992). Workers had significantly (p < 0.05) worse ratios of peak- to post-season test scores than non-worker residents in the same "exposed" kibbutzim (see Table 7.27).
Table 7.27
Difference Between Peak- and Post-Season Test Score Ratios of
Occupationally Exposed and Non-Exposed Israelis
|
Test |
|
| Digit symbol (scaled score) | -3.3 |
| Digit span backwards | -10.3 |
| Scaled score (test for short-term memory) | -10.6 |
| Symptoms (depression) | -4 |
Some cases involving household pesticide use also provide information about the nature of OP effects following prolonged exposure. Richter et al. (1992) reported that four family members were experiencing fatigue, sleep problems, irritability, vomiting (infant), runny nose (infant), dizziness, headache, and chest heaviness four and one-half months after their apartment was treated commercially with diazinon. Diethylphosphate, a urinary metabolite, was also detected in the urine of symptomatic household members four and one-half months after the diazinon application. Swabs of wall surfaces revealed residual concentrations (126 to 1,051 �g/m2) much higher than ambient air concentrations (2.5 to 0.1�g/m3) 0 to 56 days after indoor spraying. A coat and skirt that emitted an odor were also found to be contaminated. The 30-year-old mother and an infant were most affected, although symptom persistence was not reported.
Kaplan et al. (1993) presented a similar case series of residential exposure to chlorpyrifos resulting in such symptoms as cognitive slowing, cognitive problems, and sensory neuropathy weeks to months after application. Again, symptom persistence was not reported.
As discussed above, it is often difficult to attribute specific effects to either acute or chronic pesticide exposures. This difficulty can be due to inaccurate or incomplete reporting, misattribution of symptoms, or a variety of other factors. In many studies of persistent effects, acute toxicity is used to select the study group. In some cases, acute exposures great enough to cause illness (e.g., poisonings) clearly implicate the causative agent. Pesticide exposures of these types were not reported to have occurred during ODS/DS. Therefore, studies of chronic effects in the absence of acute toxicity may be of greater relevance.
Richter et al. (1992) assessed symptoms in 11 Israeli ground-crew workers who were exposed to OP and other pesticides and displayed low-level cumulative systematic reductions in ChE activity, compared with 13 controls. Test results (electroneuromyography) indicated lower peak amplitude in sural but not peroneal nerve (mean �SD = 10.1�4.0 vs. 14.0�2.6 mV, p < 0.05). The authors comment, "In all groups, evidence of exposure-illness associations was found even though persons with acute poisoning were not seen."
The same 11 subjects, age 24 to 36 years, were compared with 13 male kibbutz residents, age 24 to 41 years, living further than 1 km from a sprayed field (Richter et al., 1992). The ground-crew workers had higher frequencies (relative risks > 2) of fatigue, dizziness, concentration problems, confusion, memory problems noted by others, depression, palpitations, sleep problems, weakness in extremities, tingling in extremities, headaches at work, nausea, vision problems, cramps, breathing problems, uncontrolled sweating, "annoyance from odors at work," and urinary frequency. Controls may have been exposed, but at a lower level. Workers with past self-reported episodes of acute illness (n = 20) did no worse on tests than an age-sex-education-matched comparison group; and those who "spent their youth" in the kibbutz ("possible childhood exposure") did no worse than age-sex-education-job matched residents who joined the kibbutz after age 20 (<10 years of seasonal exposure); in fact, the former group scored higher on the Benton Visual Retention Test and the Trails 2 Test. However, strong conclusions cannot be drawn because there may be relevant differences in these populations. Those who remained in the kibbutz may have self-selected for resilience to pesticides and/or less exposure; people who chose to move from a kibbutz may have differed from those who remained in one. In addition, the samples are too small to overcome large variability in normal values. Analogously, another cited study showed that in-season variations in ChE activity were well within normal limits in 26 workers and 11 residents exposed to spray drift and seven residents who were not exposed. However, additional data showed that reductions were greater in workers and exposed residents than in unexposed residents.
An observational NIOSH study compared 45 male pesticide workers who had prior histories of documented ChE inhibition below worker removal thresholds, but no evidence of frank poisoning, with 90 subjects who were not "expected to have current cholinesterase inhibition" but were not otherwise defined, on a series of neurological tests, including neurobehavioral tests, nerve conduction tests, vibrotactile sensitivity tests, tests of postural sway, and a clinical exam (Ames et al., 1995). It was not stated whether the controls also worked with pesticides. Acute toxicity was not reported and was, in fact, used as an exclusion criterion. The study group did not perform significantly worse than the control group on any of 27 tests performed. Tests included nerve conduction velocity and amplitude (median motor and sensory, ulnar sensory, peronial motor, sural sensory); vibration (finger, toe); neurobehavioral (tapping, hand-eye, simple reaction time, sustained attention, digit-symbol, pattern memory, serial digit); mood (tension, depression, anger, fatigue, confusion); and motor coordination (pursuit aiming, Santa Ana dexterity, postural sway). Because of the nature of the study group and the fact that prior pesticide exposure in the control group was not defined, the results support the observation that degree of cholinesterase inhibition does not correlate well with presence (or development) of neurobehavioral abnormalities. The choice of subjects with no clinical symptoms despite low ChE levels may also have biased the subset toward the most physiologically resilient.
Genetic Effects
One study of 13 malathion-exposed workers in the Southern California Mediterranean fruit fly eradication program, in which malathion was used as ground treatment, examined micronucleus formation and mutation frequencies assessed by the glycophorin A (GPA assay). The workers were compared with only four controls, who supervised or organized crews and may therefore not have been unexposed (Windham et al., 1998). In a 1992 pilot project, the mean micronuclei level appeared higher in lymphocytes of exposed workers (20.1�7.1 vs. 14.3�7.2, p = 0.09), but the finding did not reach significance. In the 1993 season, an additional 24 workers and 11 controls (primarily staff "not directly involved in malathion application") were recruited. Neither the first nor the second cohort showed a higher level of micronuclei than did the presumably less-exposed control group; nor did the pooled total (means = 17.8�7.2 vs. 18.5�6.3); nor did they after adjustment by multiple regression. Glycophorin A variant frequency was not shown to be associated with malathion exposure. Of note, 29 percent of the "office workers and supervisors" who served as controls reported having been exposed to pesticides in the previous six months. In addition, 21 of the applicators had also been exposed to diazinon and dibrom.
Another study was initiated when the mother of a 12-year-old girl asked whether her child’s residential exposure to OPs could have genetically affected her ability to reproduce (Lieberman et al., 1998). Cytogenetic studies showed that lymphocytes of both the mother and the child were abnormal, and on the basis of this finding, a group of residentially exposed, OP-pesticide-poisoned subjects were evaluated. All had a clear temporal relationship between documented application of pesticides and classic OP pesticide poisoning. Eight subjects, ranging from 12 to 62 years of age, all reportedly in good health prior to domestic OP exposure lasting between one week and seven months, and all without other reported genotoxic exposures, were evaluated for chromosome aberrations and sister-chromatid exchange count per cell. Structural alterations in chromosomes were found in all of them; seven of eight subjects had chromosome aberrations outside the normal range for the laboratory, which was based on a control group of 141 subjects without known exposures and without illness; the eighth subject was reported to be "borderline." Sixty-three percent of subjects had slightly elevated levels of sister-chromatid exchanges.
A study of 61 male pesticide applicators in India (age 20 to 47) who worked in cotton fields without protective clothing were compared with a matched control group of 45 males (age 22 to 47) with no known pesticide exposure (Rupa et al., 1991). Pesticides included OPs such as malathion, methylparathion, monocrotophos, and quinalphos, as well as the pyrethroid cypermethrin and the OCs DDT and benzene hexachloride (BHC). The exposed group had a significantly higher frequency of sister-chromatid exchanges in peripheral lymphocytes than the controls (at each of three levels of exposure), with a monotonic dose-response relationship with exposure, and also showed cell cycle delay and decrease in mitotic index. For those in the highest exposure group (>20 years), the mean sister-chromatid exchanges per cell (�SD) compared with those of the controls were 10.54 � 1.81 vs. 3.57 � 1.85; and for the total exposed sample, the frequency was 8.46 � 2.85 (p < 0.05) for each. However, because non-AChE-inhibiting pesticides were included in this analysis, it is not possible to conclude that OPs or AChE-inhibiting agents contributed to this effect.
Another study similarly examined sister-chromatid exchanges in peripheral blood lymphocytes in Italian flower-industry workers exposed to mixed pesticides (De Ferrari et al., 1991). Exposures included but were not confined to OPs; also included were exposures to nitro-organic herbicides and fungicides, hydrocarbon derivative herbicides, and inorganic fungicides and insecticides. The sample consisted of 32 healthy flower-industry workers, 32 individuals exposed to pesticides and hospitalized for bladder cancer, and 31 controls. A significant increase in chromosome aberrations and sister-chromatid exchanges was measured in lymphocytes of both exposed groups. Cancer patients had rare rearrangements (dicentrics, rings, and quadriradials) at a higher rate than healthy exposed persons; these were not observed in unexposed controls. Hyperdiploid and polyploid metaphases were also significantly increased in the two exposed groups. Stratifying for age and smoking did not change the substance of the results. Once again, the group exposed to mixed pesticides had higher rates of markers suggesting increased cancer potential; however, the contribution (if any) of OPs among the mix of exposures cannot be ascertained.
Finally, a study in Denmark compared 134 pesticide-exposed greenhouse sprayers with 157 referents (Landers and Rønne, 1995). Exposures among the sprayers included carbamates (the most widely used agents were benomyl--a carbamate fungicide--and pirimicarb), OPs, polychlorinated insecticides, pyrethroids, fungicides, and growth regulators. Sister-chromatid exchanges were higher among non-smoking but not among currently smoking sprayers than among matched referents. (Both age and smoking were related to significant increases in sister-chromatid exchanges, p = 0.002 and p = 0.0005, respectively). The frequency of pesticide applications, lifetime pesticide exposure, and in-season plasma-cholinesterase inhibition did not influence the sister-chromatid exchange frequency. Once again, it cannot be determined from these data whether AChE-inhibiting agents (carbamate or OP pesticides) contributed to the greater increase in frequency among non-smoking sprayers.
In Vitro Data Using Human Lymphocytes. A host of studies have examined mutagenic behavior of OPs and carbamates in human lymphocytes in vitro (Bianchi-Santamaria et al., 1997; Bonatti et al., 1994; Cid et al., 1990; Cid and Matos, 1984; Dolara et al., 1994; Garry et al., 1990; Lieberman et al., 1998; Lopez and Carrascal, 1987; Kappas et al., 1990; Kevekordes et al., 1996; Nicholas et al., 1979; Perocco and Fini, 1980; Veronesi and Ehrich, 1993; Rupa et al., 1991; Rupa et al., 1989; Rupa et al., 1988; Sobti et al., 1982). These studies examined a variety of OPs (including microtophos, malathion, chloracetophone, parathion, azinphos-methyl, dimethoate, pirimphos-methyl, diazinon) and carbamates (methomyl, aldicarb, propoxur, benomyl), as well as pesticide combinations, and reported interference in reparative DNA synthesis; increased damage to human lymphocyte DNA and interference with DNA repair processes after damage exerted by ultraviolet rays; and OP-associated increases in mitotic index, sister-chromatid exchanges (Perocco and Fini, 1980), chromatid breaks, gaps, deletions, fragments, exchanges, dicentrics, and endoreduplications (Rupa et al., 1988). One study examined a variety of OP and carbamate compounds using a micronucleus test, with chemical doses based on subjects’ estimated daily intake. Weak genotoxicity at these doses was seen in three of the four tested OPs and in the tested carbamate (benomyl); additive effects were not seen (Bianchi-Santamaria et al., 1997). The timing of exposure of the cell culture was also found to influence the susceptibility to chromosomal aberrations (tested with diazinon) (Lopez and Carrascal, 1987).
One study used a cell cloning assay to study genotoxicity of malathion to human T lymphocytes in vitro. Cells in phase G0 were exposed to doses of malathion ranging from 10 to 600 �g/ml (Pluth et al., 1996). In seven in vitro experiments using cells from four different individuals, and one experiment in an individual exposed in vivo, one or more independent mutants containing a partial deletion of exon 3 were isolated from each individual. In five of the seven mutants, the deleted regions overlapped extensively, indicating an area within exon 3 that is exceptionally prone to deletions upon exposure to malathion. It is uncertain what the molecular mechanism is, and how this could relate to agricultural workers’ increased risk of cancer.
Neuroblastoma cell lines have also been examined (in humans and mice), and evidence suggests that interspecies selectivity in response to OP-related cytotoxicity is influenced by intercellular differences in metabolism and baseline esterase activity, as well as cytochrome-P450-associated monooxidase activity (Veronesi and Ehrich, 1993).
Studies in Microbial and Mammalian Systems. The possibility of carcinogenic effects is supported by in vitro studies demonstrating mutagenicity to bacteria, increased mammalian chromosomal damage and micronucleus formation, and sister-chromatid exchange, chromosomal aberrations, and transformation in cultured rat tracheal epithelial cells; DNA single-strand breaks in isolated rat hepatocytes; and increases in Syrian hamster embryo cell transformations and SA7 virus-induced transformations of hamster embryo cells, replicated in three different laboratories but requiring high doses of OPs in order to be cytotoxic (Mennear, 1998). Some reports suggest that the in vitro genotoxic effect may occur through direct alkylation, but in vivo metabolism of the parent molecule is thought to preclude this effect. Additional studies suggesting a genotoxic effect in mammals (usually with high dose exposures) and other studies failing to suggest such an effect have been reviewed (Mennear, 1998).
Many studies have examined the impact of OPs and carbamates on chromosomal aberrations, mutagenicity, and sister-chromatid exchanges in mammalian systems, some with positive findings and some with negative findings (Chen et al., 1981; Degraeve and Moutschen, 1984; Degraeve et al., 1978; Dulout et al., 1982; Salvadori et al., 1988; Vaidya and Patankar, 1982; Wang et al., 1987). Although one report states that "most of the organochlorinated, organophosphorus, carbamate and pyrethroid group of pesticides were reported to be positive for cytogenetic effects in mammalian systems" (Rupa et al., 1989), evidence in cell cultures has not been wholly uniform. A study coauthored by the Dow Chemical company concluded that the OP chlorpyrifos has minimal mutagenic potential (Brenner et al., 1989). Another report concluded that "the genotoxic data to date have been somewhat inconclusive with regard to malathion exposure" (Pluth et al., 1996).
It has been suggested that some of the inconsistencies, particularly in the malathion data, may result from the difference between purified malathion (>99 percent pure) and technical-grade malathion, the grade used for agricultural purposes, which is usually 90 to 95 percent pure and may contain up to 11 impurities, some of which have been found to be significantly more toxic than malathion or to potentiate the toxicity of malathion (Pluth et al., 1996; Umetsu et al., 1977; Flessel et al., 1993). In addition, malaoxon, the active metabolite of malathion, tested positive for mammalian gene mutations in instances in which it was tested (Flessel et al., 1993). Adding to the inconsistency, most studies in bacteria and yeast have failed to show a mutagenic effect (Shirasu et al., 1975; Waters et al., 1982; Wong et al., 1989; Mohn, 1973; Wild, 1975), while studies in human lymphocyte cultures have commonly shown one (Pluth et al., 1996; Herath et al., 1989; Garry et al., 1990; Walter et al., 1980; Sobti et al., 1982; Nicholas et al., 1979). Rodent studies have been variable, depending on the assay (Degraeve et al., 1984; Degraeve and Moutschen, 1984).
The relationship between genetic effects and clinical disease is the subject of current investigations. While the presence of genetic effects is not always an indicator of clinical disease, these effects are important because of their association with increased cancer risk (Hagmar et al., 1994). Some evidence has suggested that low doses of some chemicals may be more genotoxic than high doses, so extrapolation from high to low doses may be misleading (Au et al., 1990).
Reproductive Effects
Data on whether OPs may produce adverse reproductive outcomes are presently unclear. Few studies that directly evaluate this issue are available in the literature. Many studies have assessed mutagenicity of OP and carbamate pesticides, which may relate to genotoxicity; and many of these studies have suggested low-grade mutagenicity. However, there is little evidence of direct impact on reproductive outcomes.
Carcinogenic Effects
Data on both exposure and outcome are limited, and confounders remain important concerns, limiting the ability to draw epidemiological inferences. Available forms of data include
Carcinogenicity in Animal Studies. Debate continues concerning the possible carcinogenicity of some OPs in animals. An International Agency for Research on Cancer (IARC) monograph concluded that there was little evidence of strong mutagenic or carcinogenic effects in mammals from five commonly used OP pesticides (malathion, methyl parathion, parathion, tetrachlorvinphos, and trichlorfon) (International Agency for Research on Cancer, 1983), but interpretation of the underlying studies has been controversial (Minton and Murray, 1988; Huff et al., 1985; Reuber, 1981, 1985).
Among the potentially contradictory evidence are studies showing that dichlorvos causes a sex-specific, species-specific increase in a mononuclear cell leukemia: Male Fischer 344/N rats receiving up to 103 weeks of dichlorvos at either 4 mg/kg or 8 mg/kg by gavage experienced approximately twice as many cases of a mononuclear cell leukemia than those dosed only with the corn oil vehicle (p = 0.012 for 4 mg/kg; p = 0.008 for 8 mg/kg) (Mennear, 1998). No difference in rates was seen for female rats. The implications to humans of this sex- and species-specific increase are unclear. Moreover, the National Toxicology Program considered the results of the female mouse (but not the male mouse) portion of study to afford unequivocal evidence of carcinogenesis (Mennear, 1994). It has been noted that dichlorvos "possesses no in vivo mutagenic activity in mammalian assay systems, and it bears no significant structural similarity to known carcinogens," so one author considered "a weight-of-the-evidence analysis" to lead to the conclusion that dichlorvos "poses neither mutagenic nor carcinogenic risks to humans exposed under normal conditions of use or foreseeable conditions of misuse" (Mennear, 1994).
Studies of Carcinogenicity in Humans. A number of epidemiological studies report a statistically significant increase in death from hematological malignancies among persons in farming occupations (Milham, 1971; Cantor, 1982; Blair et al., 1985; Hoar et al., 1986; Brown et al., 1990; Pasqualetti et al., 1991). It cannot be presumed that AChE-inhibiting OP and carbamate pesticides are necessarily responsible for these findings, but increased mutagenicity and genotoxicity of OP and carbamate agents to human lymphocytes in vitro (data reviewed previously) take on new significance in light of these reports.
Leukemia and Lymphoma. Data regarding whether OP pesticides are risk factors for leukemia remain inconclusive. Many studies have demonstrated an apparent increased risk of lymphoma and leukemia in farmers (Brown et al., 1990), and some have shown an apparent increased odds ratio (OR), or crude odds ratio, for leukemia (Brown et al., 1990) and lymphoma (Persson et al., 1993). Although in some cases the significance of the effect is lost following adjustment for other factors (Persson et al., 1993), it is difficult to know whether those factors might be merely correlated with pesticide use rather than causal in a fashion that causes adjustment to "adjust out" a true effect. In a population-based case-control study in Iowa and Minnesota of 578 white men with leukemia and 1,245 controls, "significantly elevated risks for leukemia of �2.0" were seen for exposure to the OPs crotoxyphos (OR = 11.1), dichlorvos (OR = 2.0), and lamphur (OR = 2.2), as well as the natural product pyrethrins (OR = 3.7) and the chlorinated hydrocarbon methoxychlor (OR = 2.2) (Brown et al., 1990). Clearly, additional research is warranted to assess the relationship between leukemia and lymphoma and OP (and perhaps carbamate) exposure.
Non-Hodgkin’s Lymphoma. Some studies suggest a relationship between pesticides, including OPs and carbamates, and non-Hodgkin’s lymphoma (NHL), a tumor whose incidence rates have increased worldwide in both men and women for 30 years (Milham, 1971; Cantor, 1982; Blair et al., 1985; Hoar et al., 1986; Brown et al., 1990; Pasqualetti et al., 1991).
Lung Cancer. Some studies of male pest-control workers have reported an increased risk of lung cancer linked to the number of years subjects have been licensed. One study reported a standardized mortality ratio (SMR) of 1.4 from lung cancer in pest-control workers in Florida, rising to 2.9 among workers employed for more than 20 years (Blair et al., 1983). This study was limited by lack of information on smoking status and on specific pesticides. In an effort to redress these defects, a nested case-control study was performed to determine the relation of smoking and type of pesticide to risk, comparing 65 deceased lung cancer cases, 122 deceased controls, and 172 living controls, using information obtained from interviews with next of kin for both living and dead subjects. ORs for lung cancer were 2.4 (95 percent CI = 1.0-5.9) for deceased controls and for workers first licensed before age 40, and increased from 1.4 (95 percent CI = 0.7-3.0) for those licensed 10 to 19 years to 2.1 (95 percent CI = 0.8-5.5) for those licensed 20 or more years. The risk of cancer was greater among pest-control operators than among non-pest-control operators. (The increase in risk was not significant among the living controls: OR = 1.5, 95 percent CI = 0.6-3.3.) Although small, the lung cancer risk among pest-control operators appeared to be possibly associated with reported exposure to carbamates (OR = 16.3, 95 percent CI = 2.2-122.5, dead controls), OPs (OR = 2.2, 95 percent CI = 0.8-5.5, dead controls), and phenoxyacetic acids (OR = 4.8, 95 percent CI = 0.6-35.5, dead controls); and to the specific OP diazinon (OR = 2.0, 95 percent CI = 0.7-5.5, dead controls) and the specific carbamates carbaryl (OR = 4.2, 95 percent CI = 0.6-27.2, live controls; data not given for dead controls) and propoxur (OR = 12.4, 95 percent CI = 1.5-100.3, dead controls; OR = 1.4, 95 percent CI = 0.4-5.5, live controls). OR estimates were lower when living controls were used, except in the case of phenoxyacetic acids. Compared with the general U.S. mortality experience, overall mortality was not elevated, although lung-cancer-specific mortality was increased.
Carbamates have the same presumed primary mechanism of toxicity as OPs; that is, they are both AChE inhibitors. Thus, even though OPs inhibit AChE irreversibly (requiring more enzyme to be produced for function to be restored), whereas carbamates inhibit AChE reversibly, OPs and carbamates are often considered together (Lerman et al., 1984; D'Mello and Sidell, 1991; Bardin et al., 1994). The literature regarding the acute and chronic effects of carbamates has largely been reviewed in the previous sections.
There are two major classes of pesticidal carbamates (Miller 1982). The first class is ChE-inhibiting carbamates, including monomethylcarbamates and dimethylcarbamates. These are used primarily as insecticides (both contact and systemic) and also as miticides, rodenticides, nematocides, anthelmintics, and molluscides; in addition, they are used for treatment of glaucoma and myasthenia gravis, and as antagonists for curare or curarimimetic poisoning (Miller, 1982). The methylcarbamates and dimethylcarbamates inhibit ChE by carbamylation of the esteratic site of the enzyme; and in the case of AChE, they prevent the enzyme from de-esterifying ACh. Some selectively inhibit either "true" cholinesterase (RBC AChE or simply AChE, found in red blood cells and nervous tissue) or butyrylcholinesterase (BuChE, or psuedocholinesterase or plasma cholinesterase) or both; others selectively inhibit peripheral ChEs (Miller, 1982). Cholinesterase-inhibiting carbamates are often referred to as "reversible ChE inhibitors," although this designation has been criticized on the grounds that it suggests that they dissociate from the enzyme intact, whereas they are covalently bound to the active site of the enzyme and are typically hydrolyzed in the same manner as is acetylcholine (Miller, 1982, citing 39, 57). These carbamates can be biotransformed through any of several metabolic mechanisms, including N-demethylation, aromatic ring hydroxylation, O-dealkylation, alkyl hydroxylation, and sulfoxidation; however, hydrolysis of the carbamate moiety is the major route of metabolism. In instances in which biotransformation does not involve separation of this ester bond, the metabolic products may also be ChE inhibitors and may be more potent than the parent compound (Miller, 1982).
The other major class is the dithiocarbamates, sulfur-containing carbamates which may have little or no esterase-inhibiting action and which are often, but not exclusively, used as fungicides and herbicides. These include methyldithiocarbamates, dimethyldithiocarbamates, diethyldithiocarbamates, and ethylenebisdithiocarbamates. These are highly reactive due to their metal-combining capacity and their ability to interact with sulfhydryl-containing compounds. The pesticides of concern identified by OSAGWI do not include dithiocarbamates, and this class is not discussed further in this report.
Hepatitis has been reported to have followed exposure to carbamates, specifically pyricarbate (pyridinol carbamate) (See and Bouvry, 1984; Grange et al., 1984). Carbamates are not known to produce OPIDN or intermediate syndrome in the absence of OPs; they may, however, cause these syndromes to occur when they otherwise might not if carbamate exposure occurs after exposure to an OP that may cause OPIDN or intermediate syndrome.
Genetic Effects
Information on genetic effects of carbamates was reviewed in the previous section, where some cited studies evaluated both OP and carbamate agents.
As stated previously, "most of the organochlorinated, organophosphorus, carbamate and pyrethroid group of pesticides were reported to be positive for cytogenetic effects in mammalian systems" (Rupa et al., 1989), although findings are not consistent.
Studies have included carbamates (e.g., methomyl, aldicarb, propoxur, benomyl) as well as pesticide combinations. One study examined a variety of OP and carbamate compounds using a micronucleus test, with chemical doses based on subjects’ estimated daily intake. It found weak genotoxicity at these doses for three of the four tested OPs and for the tested carbamate (benomyl) (Bianchi-Santamaria et al., 1997).
Reproductive Effects
Little independent information on the reproductive effects of carbamates was reviewed. High dose rates of the carbamate fungicide benomyl have been linked to eye defects (including anophthalmia) in humans and to reproductive effects in animals (Handysides, 1993; Watterson, 1994), but these findings do not appear to extend to other carbamates.
Carcinogenic Effects
Few data directly relating carbamate exposure to cancer in humans have been reported.
In a nested case-control study (described above) that compared 65 deceased lung-cancer cases, 122 deceased controls, and 172 living controls, using information obtained from interviews with next of kin for both living and dead subjects, lung cancer risk among pest-control operators appeared to be possibly associated with reported exposure to carbamates (OR = 16.3, 95 percent CI = 2.2-122.5, dead controls) and to the specific carbamates carbaryl (OR = 4.2, 95 percent CI = 0.6-27.2, live controls; data not given for dead controls) and propoxur (OR = 12.4, 95 percent CI = 1.5-100.3, dead controls; OR = 1.4, 95 percent CI = 0.4-5.5, live controls). OR estimates were lower when living controls were used. Compared with the general U.S. mortality experience, overall mortality was not elevated, although lung-cancer-specific mortality was increased.
Additional data relating cancer to carbamate exposure, as part of pesticide exposure more generally, are reviewed in the section discussing OPs and cancer.
Data pertaining to genetic effects of carbamates, which may pertain to cancer risk insofar as mutagenicity relates to carcinogenicity, are presented in the section on OPs and genetic effects, where some of the reviewed studies describe effects of carbamates as well as OPs. Carbamates have been shown to have genetic effects similar in character to those observed with OPs.
Symptoms found to occur following exposure to AChE inhibitors such as OP and carbamate pesticides include fatigue, joint and muscle symptoms, sleep effects, headaches, skin effects, cognitive effects, mood effects, and neurological effects. These classes of symptoms are also seen frequently in ill PGWV--they are among the most frequent principal diagnoses in the PGW registry (see Chapter Three). This observed similarity in symptoms of ill PGWV and persons exposed to AChE inhibitors is not sufficient in itself to conclude that OP and carbamate pesticide exposure is a cause of the myriad health problems reported by PGWV. However, we believe it is inappropriate at this point to conclude that such exposures played no role. In the absence of better information about such exposures, the similarity in symptoms is sufficient to consider pesticide exposures among the potential causes of undiagnosed illnesses in PGWV that cannot be eliminated on the basis of a review of published evidence to date.
Comparatively few studies directly assess the impact of short-term AChE-inhibitor exposure, without acute toxicity, on long-term symptoms and neuropsychological and neurobehavioral outcomes. However, there is evidence of modest long-term effects on these outcomes following AChE-inhibitor exposures that were insufficient to lead to acute symptoms or medical attention, and there is tentative evidence to suggest that these findings may occur selectively in persons who experience more-pronounced symptoms on acute exposure (Beach et al., 1996). There is also evidence of modest long-term effects on cognitive outcomes in ill PGWV.
Additional comprehensive evaluations of larger samples of subjects previously exposed to OP and carbamate pesticides, including those who did and did not experience symptoms on acute exposure and others whose period of exposure was short, would be useful in predicting possible specific effects in ill PGWV if AChE-inhibitor exposures are found to be a contributor to undiagnosed illnesses among this population.