Investigators quantified both application and post-application exposure to DEET under Post-Application Scenarios below. Investigators did not quantify application exposure alone because they judged it to be inconsequential.
Up to about 44% of servicemembers in the RAND survey (Table 8) indicated that they used permethrin or witnessed its use. However, the value of 44% is probably biased somewhat high, as the product was identified as "personal-use spray," and probably included a minor proportion of other products. Forty percent of the PM interviews cited use of permethrin, 0.5% aerosol. The 0.5% aerosol formulation, in a 6-oz spray can, was the most common form, and is the one evaluated in this exposure assessment. Permethrin was applied mainly to battle-dress uniforms (BDUs), and to a lesser extent to tents and bed nets. The spray was to be applied to clothing while it was hanging up then allowed to dry for 2 to 4 hours before being worn. The formulation in the can is a mixture of the active ingredient, and various inert ingredients such as solvents and/or propellants.
An important determinant of the level of absorbed pesticide active ingredient dose is the level of PPE worn by applicators. The PM interviews indicate that some servicemembers wore PPE; however, only 14% of servicemembers who identified permethrin addressed PPE. It is probable that the majority of servicemembers did not wear PPE when applying permethrin, as it would not normally have been required, unless someone was assigned the task of spraying many sets of BDUs. Thus, investigators evaluated the following clothing scenarios for servicemembers applying permethrin:
Low exposure: long pants, long-sleeve shirt, gloves, half-face respirator with organic vapor cartridges and pesticide prefilters (respiratory protection factor = 90%).[157]
Medium and high exposure: long pants, long-sleeve shirt ("single layer, no gloves" from the PHED Guide).
Table 23 presents the assumptions for application of permethrin, 0.5% aerosol. As shown in Table 13 from the PM interviews, 26% of the servicemembers who identified permethrin aerosol indicated that it was used mainly outdoors, while 9% indicated that it was used mainly indoors; 65% did not provide information. The label directed the user to make all applications outdoors; thus, indoor application was a misuse.[158]
Table 23. Permethrin assumptions for application
| Factor | Units | Definition/Explanation | Assumptions by Level | Source/Rationale |
||
| Low | Medium |
High |
||||
| UE | mg/lb a.i. |
Unit dermal exposure | 81 |
190 |
190 |
1998 PHED Guide: Aerosol[159] |
| UIE | mg/lb a.i. |
Unit inhalation exposure | 0.13 |
1.3 |
1.3 |
1998 PHED Guide: Aerosol[160] |
| WA | lb a.i./d |
Weight of a.i. handled | 0.0019 |
0.0019 |
0.0019 |
Weight of a.i. in one 6 oz can of 0.5% formulation;a assumes 1 can/day for each day used |
| EF | d/mo |
Exposure frequency | 2 |
4 |
8 |
XVIII Airborne[161] |
| ED | mo |
Exposure duration | 1 |
4 |
8 |
PM interviews, Table 13 |
| ABS | -- |
Dermal absorption factor | 0.02 |
0.02 |
0.02 |
NRC[162] |
| a) | (6 oz) x (0.005) x (1 lb/16 oz) = 0.0019 lb a.i. in one 6 oz can. |
c. Personal-Use Products Doses — Application
Investigators did not evaluate application exposure separately for DEET. Table 24 presents doses potentially resulting from application exposure to permethrin, 0.5% aerosol. There are three types of doses presented for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. Table 25 presents the doses for the evaluation of potential carcinogenic effects for permethrin due to exposure during application. The two types of doses shown are LADDD and LADDI.
Table 24. Permethrin, dose rates – application, for evaluation of noncarcinogenic effectsa
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRD |
ADD |
PDRI |
|||||
| Permethrin, 0.5% aerosol | Low | -- |
0.02 |
2.20E-03 |
4.40E-05 |
3.53E-06 |
|||||
| Medium | -- |
0.02 |
5.16E-03 |
1.03E-04 |
3.53E-05 |
||||||
| High | -- |
0.02 |
5.16E-03 |
1.03E-04 |
3.53E-05 |
||||||
|
|||||||||||
| a) | ABS = dermal absorption factor. |
| PDRD = potential dose rate for dermal contact. | |
| ADD = absorbed dermal dose. | |
| PDRI = potential dose rate for inhalation. | |
| UE = unit dermal exposure | |
| WA = mass of a.i. | |
| BW = body weight. | |
| UIE = unit inhalation exposure. | |
| b) | Formulas 1 and 3 adapted from EPA.[163] |
Table 25. Permethrin, lifetime average daily doses – application, for evaluation of carcinogenic effectsa
Formulation |
Exposure Group |
Exposure Point |
LADDD (mg/kg/d) |
LADDI |
||||
| Permethrin, 0.5% aerosol | low | -- |
-- |
-- |
||||
| medium | -- |
6.46E-08 |
2.21E-08 |
|||||
| high | -- |
-- |
-- |
|||||
|
||||||||
| a) | LADDD = lifetime average daily absorbed dose via dermal contact. |
| LADDI = lifetime average daily potential dose via inhalation. | |
| A dash ("--") indicates that the item is not applicable. | |
| ADD = absorbed dermal dose. | |
| EF = exposure frequency. | |
| ED = exposure duration. | |
| AT = averaging time. | |
| PDRI = potential dose rate for inhalation. | |
| b) | Formulas adapted from EPA, 1997.[164] |
The RAND survey determined 50% of servicemembers used DEET or witnessed others using DEET. Thirty-one percent of the PM interviews cited DEET, 33% stick/cream, while 18% cited DEET, 75% liquid. The DEET preparations used were similar to those widely available to the American public for many years. Servicemembers applied DEET to the skin as a repellent for numerous arthropods, including sand flies, mosquitoes, ticks, fleas, and mites. For purposes of this assessment, investigators assumed the conditions of use for DEET, 33% stick/cream are identical to those for DEET, 75% liquid. The only difference in the scenarios, exposure factors, etc. is the active ingredient concentration in the formulation.
Table 26 presents the assumptions for DEET post-application exposure. In this analysis, investigators considered application and post-application together. In reality, "application" is completed within a few minutes, and then post-application exposure continues for hours, until either all active ingredient evaporates, is absorbed, or is removed from the skin surface (e.g., while bathing). The only relevant exposure route is the dermal route. In a recent exposure assessment, EPA quantified exposure via the dermal route, but not via oral or inhalation routes.[165] Little if any DEET is likely to be ingested by adults. A small amount of DEET may be inhaled under some circumstances; however, EPA does not provide an inhalation toxicity value with which to evaluate inhalation exposure (see Section B.4, Toxicity Assessment)
Table 26. DEET assumptions for post application
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale | ||
Low |
Medium | High | ||||
| CS1 | mg/kg |
Concentration of a.i. in the stick/cream formulation | 330,000 |
330,000 |
330,000 |
33% = 330,000 mg/kg |
| CS2 | mg/kg |
Concentration of a.i. in the liquid formulation | 750,000 |
750,000 |
750,000 |
75% = 750,000 mg/kg |
| CF | kg/mg |
Unit conversion factor | 1E-06 |
1E-06 |
1E-06 |
Standard |
| N | d-1 |
Number of applications | 1 |
2 |
7 |
Survey (Table 9)a |
| SA | cm2 |
Skin surface area available for contact | 5,000 |
5,000 |
5,000 |
EPA[166,167] |
| AR | mg/cm2 |
Application rate of formulation | 1 |
1 |
1 |
EPA[168] |
| EF | d/mo |
Exposure frequency | 4 |
15 |
24 |
Survey (Table 9)a |
| ED | mo |
Exposure duration | 2 |
4.5 |
8 |
PM Interviews (Table 13)b |
| ABS | -- |
Dermal absorption factor | 0.2 |
0.2 |
0.2 |
EPA[169] |
| a) | Values for 5th, 50th, and 95th percentiles, respectively. |
| b) | Values for 10th percentile, average, and 90th percentile, respectively. Comparing the ED data for 33% and 75% DEET from Table 13, the 10th percentile values are the same, and the 90th percentile values are the same. The average values from Table 13 were averaged to obtain 4.5 months. |
Table 26 presents the source and rationale for each exposure factor. The application rate (AR) in units of mg formulation per cm2 listed is based on input from EPA. EPA described a study by the DEET registrant where adults were asked to apply DEET with varying percentages of active ingredient.[170] The registrant is the person or company attempting to register a pesticide product with the EPA. The mean amount of active ingredient per application was 925.25 mg active ingredient and 649.31 mg active ingredient for males and females, respectively. If one assumes the 925.25 mg active ingredient is applied over 5,000 cm2 of skin surface area, then the AR for the study described is about 0.18 mg a.i./cm2. Based on the latter result, one can estimate ARs of 0.55 mg and 0.24 mg formulation per cm2 for the 33% and 75% formulations, respectively. Thus, the AR listed in Table 26 of 1 mg formulation per cm2 is certainly conservative, being about 2-4 times that based on the study submitted by the registrant.
Table 27 presents the assumptions for post-application exposure to permethrin, 0.5% aerosol. The label for a 6-oz can provides the following directions for use: "Treat the clothing for a minimum of 30 seconds on each side ... Use approximately � of this container to treat one complete field uniform. Use remainder on mosquito netting."[171] Investigators assumed that 90% of the sprayed active ingredient adheres to the clothing, while the remaining 10% becomes suspended in the indoor air. Post-application dermal exposure occurred daily by migration of permethrin from the treated BDU to the skin. Inhalation exposure occurred for 8 hours in tents where servicemembers treated BDUs. Investigators assumed indoor application, a misuse of the product, occurred fairly infrequently for low-exposure and medium-exposure receptors, but more frequently for high-exposure receptors.
Table 27. Permethrin assumptions for post application
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale | ||
Low |
Medium | High | ||||
| WA | g |
Mass of a.i. | 0.85 |
0.85 |
0.85 |
Mass of a.i. in one 6 oz can of 0.5% formulation;a product label[172] |
| SA | cm2 |
Skin surface contact area | 17,000 |
17,000 |
17,000 |
EPA[173] |
| CS | mg/cm2 |
Concentration of a.i. in a BDU | 0.045 |
0.045 |
0.05 |
See noteb |
| MF | d-1 |
BDU-to-skin migration factor | 0.0049 |
0.0049 |
0.0049 |
NRC;[174] EPA[175] |
| ET | h/d |
Exposure time | 8 |
8 |
8 |
See notec |
| EFD | d/mo |
Exposure frequency for dermal contact | 30 |
30 |
30 |
Assumes the BDU was a constant source |
| EFI | d/mo |
Exposure frequency for inhalation. | 1 |
5 |
15 |
See noted |
| ED | mo |
Exposure duration | 1 |
4 |
8 |
PM interviews, Table 13 |
| CA | mg/m3 |
Concentration of a.i. in air | 0.0106 |
0.0146 |
0.0259 |
Air modeling; 8-hour averages |
| ABS | -- |
Dermal absorption factor | 0.02 |
0.02 |
0.02 |
NRC[176] |
| a) | (6 oz) x (0.005) x (28.35 g/oz) = 0.85 g. |
| b) | For low and medium exposure: (0.9) x (0.85 g/17,000 cm2) x (1,000 mg/g) = 0.045 mg/cm2. The factor of 0.9 assumes that 90% of the permethrin sprayed adheres to the BDU. For high exposure it is assumed that 100% adheres to the BDU. |
| c) | Assumed time in GP medium tent immediately following permethrin application. |
| d) | Assumes that one can per day is discharged in a given tent for the EFI listed. |
Investigators assumed that one 6-oz can of permethrin was discharged per day each day it was used at the exposure frequency (EFI) listed in Table 27. Indoor application was evaluated because 10% of the PM personnel said permethrin was used mainly indoors, and 65% did not respond. To be conservative, investigators assumed that at least a portion of the 65% represented additional cases where permethrin was applied mainly indoors.
c. Air Modeling for Permethrin
Investigators conducted air modeling to estimate the permethrin emissions and indoor air vapor concentrations resulting from treatment of one BDU inside a GP medium tent. A soldier sprays his/her uniform as directed, and other servicemembers in the tent receive a secondary exposure to the spray during this treatment and after the spraying is completed. The secondary exposure continues until the suspended permethrin is removed by ventilation or until servicemembers leave the tent. This secondary exposure to permethrin is considered in the air modeling analysis. For air modeling purposes, investigators assumed that an entire can is used to treat one uniform and that the treatment takes approximately 10 minutes to complete. Investigators also assumed that 10% of the contents of the can becomes suspended in the indoor air. Investigators assumed the airborne permethrin to be either vapor or fine mist (very small aerosol droplets that behave and disperse similarly to vapor).
1) Permethrin Emission Calculation
Investigators calculated the emission rate of permethrin to the indoor air according to the following mass balance equation:[177]
E = S x p x f x 1,000 / t
where,
E = emission rate, mg/min
S = amount of pesticide formulation applied, grams
p = fraction by weight of active ingredient in pesticide formulation, unitless
f = fraction of pesticide formulation which becomes airborne, unitless
t = duration of application, minutes
Based on the manufacturer’s label information and the assumption that the entire application takes 10 minutes to complete, investigators calculated the emission rate from treatment of one uniform as follows:
E = 8.505 mg/min
S = 6 oz = 170.1 g
p = 0.005 (0.5%)
f = 0.10 (10%)
t = 10 min
2) Calculating Indoor Air Concentrations
Investigators calculated indoor air concentrations resulting from a single application event using a standard box model approach assuming complete mixing. The derivation of the box model is detailed below.
Investigators calculated indoor air concentrations in two phases. The first phase considers concentrations during the duration of the application (10 min). The second phase considers concentrations during the remainder of the 8-hour period following the onset of the application. Investigators calculated the average concentration during this period based on the average concentration during each phase (weighted by the duration of each phase).
One can develop the box model equation from mass balance considerations. The rate of change in the mass of active ingredient in the air inside the room is equal to:
the rate of emission of active ingredient to the air
plus the rate of transport of active ingredient from the outside
minus the rate of transport of active ingredient from the room to the outside
minus the rate of decay of active ingredient inside the room
One can write this in the form of a differential equation, which describes the change in concentration over time:
V(dC/dt) = E + CaIV – CIV – KCV
where:
C = concentration (mg/m3)
Ca = ambient (outdoor) concentration (mg/m3)
E = emission rate (mg/min)
I = air changes per minute in room
V = room volume (m3)
t = time (min)
K = decay rate (min-1)
This equation has the following general solution:
C = [1/(I+K)][(E/V) + (Ca)(I)][1 – exp{-(I+K)(t)}] + Co exp{-(I+K)(t)}
where:
Co = initial concentration in room (mg/m3)
Consistent with EPA draft guidance for conducting residential exposure assessments, the investigators assumed: 1) the active ingredients to be nonreactive (K = 0), and 2) contributions from outdoors to be negligible (Ca = 0).[178] Outdoor application of permethrin near tents almost certainly occurred. However, since conditions were usually very windy, the small volumes discharged would have been rapidly and extensively diluted, and the assumption of negligible contribution from outdoor to indoor air is justified.
With these assumptions, the equation for concentration within the room simplifies to:
C = [E/(I)(V)][1 – exp{-(I)(t)}] + Co exp{-(I)(t)}
For an initial concentration of zero (Co = 0), this equation for concentration at time t simplifies to the following expression:
C = [E/(I)(V)][1 – exp{-(I)(t)}]
Investigators applied this equation to calculate concentrations in the room during the assumed 10-minute period when spraying of the uniform occurred (i.e., during Phase I). The concentration in the room at the end of the application period is given by:
C10 = [E/(I)(V)][1-exp{-(10)(I)}]
One obtains the average concentration over the time interval from t1 to t2 by integrating the concentration equation over the interval and dividing by the duration of the interval:
Cavg = [1/(t2-t1)][E/(I)(V)][(t2-t1) + (1/I)(exp{-(I)(t2)} – exp{-(I)(t1)})]
The average concentration over the first 10 minutes (t2 = 10 and t1 = 0) is then given by:
CI = (1/10)[E/(I)(V)][10 + (1/I)(exp{-(10)(I)} – 1)]
For Phase II exposure (following the end of application), the initial concentration (Co) at the beginning of Phase II is the same as the concentration at the end of Phase I. Returning to the general box model equation presented earlier and again setting Ca = 0 and K = 0, the concentration in the room during Phase II is given by the expression:
Ct* = Co exp{-(I)(t*)}
where,
t* = time (in minutes) following the end of application
C0 = [E/(I)(V)][1-exp{-(10)(I)}]
One can obtain the average concentration during Phase II over the interval from t1* to t2* by integrating this equation over the interval and by dividing by the duration of the interval:
Cavg = [Co/(t2*-t1*)][-1/I][exp{-(I)(t2*)} – exp{-(I)(t1*)}]
Setting t1* = 0 and t2* = 470, the average concentration in the 470 minutes following the end of the application (i.e., during Phase II) is given by:
CII = [Co/(470)][-1/I][exp{-(470)(I)} – 1]
The average concentration over the entire 8-hour period encompassing the 10 minutes of application (Phase I) and the remainder of the period following application (Phase II) is then given by:
Cavg = (1/480)[(10)(CI) + (470)(CII)]
Investigators calculated low, medium, and high permethrin exposure levels. The three cases differ only in the fresh air infiltration rates investigators assumed for the tent. Investigators assumed ventilation rates of 6, 4, and 2 air changes per hour for the low, medium, and high indoor exposure scenarios. Investigators believe these rates are reasonable given reports that strong winds readily penetrated the tents. The air volume of the tent was set to 123 m3 to correspond to that for a GP medium tent.
Although EPA recommends the use of the Multi-Chamber Concentration and Exposure Model (MCCEM)[179] in conducting high-end exposure assessments, investigators used the simple box model equations instead for several reasons. The tent is treated as a single chamber structure, so investigators did not need the multi-chamber capability of MCCEM. In addition, the default ventilation rates included in MCCEM were not realistic for the structures and scenarios under consideration. Nonetheless, the basic physics embodied in the box model equation and in MCCEM are the same.
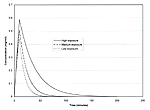
Figure 10 displays time plots of the concentration within the tent for each of the three exposure scenarios that were modeled. For each scenario, concentrations rise rapidly from zero and reach a peak value after ten minutes when the spraying ends. Following the end of application, concentrations fall exponentially and asymptotically approach zero. Table 28 presents a summary of the calculation of estimated concentrations for the application and post-application periods.
Figure 10. Time plots of permethrin air concentrations
Table 28. Calculation of indoor air concentrations for permethrin, 0.5%
Exposure scenario |
High |
Medium |
Low |
| Emission rate (mg/min) | 8.505 |
8.505 |
8.505 |
| Duration of application (min) | 10 |
10 |
10 |
| Duration of assumed exposure (min) | 480 |
480 |
480 |
| Tent volume (m3) | 123 |
123 |
123 |
| Air exchange rate (air changes/hour) | 2 |
4 |
6 |
| Air exchange rate (air changes/min) | 0.0333 |
0.0667 |
0.1 |
| Ventilation rate (m3/min) | 4.1 |
8.2 |
12.3 |
| ffff | ffff | ffff | ffff |
| Phase I (Application Period) | ffff | ffff | ffff |
| Concentration at end of application (mg/m3) | 0.5880 |
0.5047 |
0.4371 |
| Average concentration during Phase I (mg/m3) | 0.3103 |
0.2802 |
0.2544 |
| ffff | ffff | ffff | ffff |
| Phase II (Post-application Period) | ffff | ffff | ffff |
| Concentration at beginning of Phase II (mg/m3) | 0.3103 |
0.2802 |
0.2544 |
| Duration of Post-application exposure (min) | 470 |
470 |
470 |
| Average concentration during Phase II (mg/m3) | 0.0198 |
0.0089 |
0.0054 |
| Average concentration over duration of exposure (mg/m3) | 0.0259 |
0.0146 |
0.0106 |
Table 29 summarizes the average permethrin concentrations estimated within the tent for each modeled scenario for the assumed 8-hour exposure period following the onset of spraying.
Table 29. Permethrin air modeling results
Case |
Scenario |
8-hour Average Concentration(mg/m3) |
1 |
Low exposure |
0.0106 |
2 |
Medium exposure |
0.0146 |
3 |
High exposure |
0.0259 |
d. Personal-Use Products Doses — Post Application
Table 30 presents the doses potentially resulting from post-application (and application) exposure to DEET. As noted previously, only the dermal exposure route is relevant for DEET; thus, two types of doses are presented in Table 30 for the evaluation of noncarcinogenic effects: PDRD and ADD. EPA has not associated DEET with carcinogenic activity (see Section B.4, Toxicity Assessment), so investigators did not calculate LADDs.
Table 30. DEET, dose rates – post application, for evaluation of noncarcinogenic effectsa
Formulation |
Exposure Group |
Exposure Point |
ABS |
PDRO (mg/kg/d) |
PDRD (mg/kg/d) |
ADD (mg/kg/d) |
PDRI (mg/kg/d) |
||||
| DEET, 33% stick/cream | Low | -- |
0.2 |
-- |
2.36E+01 |
4.71E+00 |
-- |
||||
| Medium | -- |
0.2 |
-- |
4.71E+01 |
9.43E+00 |
-- |
|||||
| High | -- |
0.2 |
-- |
1.65E+02 |
3.30E+01 |
-- |
|||||
| DEET, 75% liquid | Low | -- |
0.2 |
-- |
5.36E+01 |
1.07E+01 |
-- |
||||
| Medium | -- |
0.2 |
-- |
1.07E+02 |
2.14E+01 |
-- |
|||||
| High | -- |
0.2 |
-- |
3.75E+02 |
7.50E+01 |
-- |
|||||
|
|||||||||||
| a) | A dash ("--") indicates that the item is not applicable |
| ABS = dermal absorption factor | |
| PDRD = potential dose rate for dermal contact | |
| ADD = absorbed dermal dose | |
| CS = a.i. concentration in the formulation | |
| CF = conversion factor | |
| N = number of applications per day | |
| SA = skin surface area available for contact | |
| AR = skin application rate | |
| BW = body weight |
Table 31 presents doses potentially resulting from post-application exposure to permethrin, 0.5% aerosol. There are three types of doses presented for the evaluation of noncarcinogenic effects: PDRD, ADD, and PDRI. Table 32 presents the application doses for the evaluation of potential post-application carcinogenic effects for permethrin. The two types of doses shown are LADDD and LADDI.
Table 31. Permethrin, dose rates – post application, for evaluation of noncarcinogenic effectsa
Formulation |
Exposure |
Exposure |
ABS |
PDRD |
ADD |
PDRI |
|||||
| Permethrin, 0.5% aerosol | Low | -- |
0.02 |
5.36E-02 |
1.07E-03 |
1.94E-03 |
|||||
| Medium | -- |
0.02 |
5.36E-02 |
1.07E-03 |
2.67E-03 |
||||||
| High | -- |
0.02 |
5.95E-02 |
1.19E-03 |
4.74E-03 |
||||||
|
|||||||||||
| a) | A dash ("--") indicates that the item is not applicable. |
| ABS = dermal absorption factor. | |
| PDRD = potential dose rate for dermal contact. | |
| ADD = absorbed dermal dose. | |
| PDRI = potential dose rate for inhalation. | |
| CS = concentration of a.i. in a BDU. | |
| MF = BDU-to-skin migration factor. | |
| SA = surface area available for dermal contact. | |
| BW = body weight. | |
| CA = concentration of a.i. in air. | |
| IRA = inhalation rate. | |
| ET = exposure time (mess and latrine). | |
| b) | Formula 1 is adapted from EPA.[180] |
Table 32. Permethrin, lifetime average daily doses – post application, for evaluation of carcinogenic effectsa
Formulation |
Exposure Group |
Exposure Point |
LADDD |
LADDI |
||||
| Permethrin, 0.5% aerosol | Low | -- |
-- |
-- |
||||
| Medium | -- |
5.03E-06 |
8.36E-06 |
|||||
| High | -- |
-- |
-- |
|||||
|
||||||||
| a) | LADDD = lifetime average daily absorbed dose via dermal contact. |
| LADDI = lifetime average daily absorbed dose via inhalation. | |
| A dash ("--") indicates that the item is not applicable. | |
| ADD = absorbed dermal dose. | |
| EFD = exposure frequency for dermal contact. | |
| EFI = exposure frequency for inhalation. | |
| ED = exposure duration. | |
| AT = averaging time. | |
| b) | Formulas adapted from EPA.[181] |
| First Page | Prev Page | Next Page |