A. Acute Health Effects
1. Oral Exposure
Common effects of acute lindane poisoning are conjunctivitis, nausea, restlessness, headache, dizziness, and vomiting with muscle tremors, abdominal pain, and diarrhea. Severe poisoning may result in seizures, convulsions, coma, and death.[906] Symptoms of acute exposure in humans usually occur within 1 hour of the dose.[907]
Research professionals apparently disagree on the lethal dose of lindane. Some have stated that 10–20 mg/kg can be lethal to humans but higher concentrations can be tolerated.[908] Others have estimated the lethal dose to be about 3 g.[909] This disparity most likely is due to variations of physical and physiological characteristics in humans.
Ohly (1973) reported a suicide attempt by a person who ingested the equivalent dose of 309 mg/kg. The individual suffered severe convulsions within the first 24 hours of ingesting the pesticide. The poisoning caused severe toxic damage to the liver, but the patient fully recovered after 5 weeks of treatment.[910]
A National Institutes of Health (NIH) Toxnet publication referenced a study in which humans tolerated a dose of 40 mg lindane per day for 14 days with "no ill effects."[911] Because of an inability to obtain the cited peer-reviewed reference, it is difficult to determine what "no ill effects" means. This statement could refer to cholinesterase inhibition levels or clinically observed symptoms. In order to be conservative, it will be assumed that the reference refers to observable frank human health effects. There is also uncertainty because of a lack of information on dose administration data and size of the test group. With these limitations in mind and a lack of other published human dose response data, the 40-mg/d dose will be used in the comparative Health Risk Assessment (HRA). Using the 40-mg/d dose and assuming oral dose administration to a 70-kg adult, the human no-observed-effect-level (NOEL) would be 0.571 mg/kg/d.
2. Dermal Exposure
Humans may be exposed directly to lindane through using medicinal preparations that are used to treat lice, scabies, and other similar skin parasites. Side effects from medicinal treatment occur mainly when the lindane treatment is misused or inappropriately prescribed.[912] However, there have been cases where neurotoxicity occurred despite proper treatment.
In a case of acute lindane poisoning, a 24-year-old woman suffered nonstop, uncontrolled body movements and visual hallucinations shortly after using lindane shampoo. The woman also was agitated, displayed constant athetotic writhing, involuntary movement, and multiple blisters over arms and legs and external genitalia. The woman had used two successive treatments of lindane shampoo (2 oz followed by 1 oz) in the course of 1 hour. The concentration of lindane in the shampoos was not reported. The lindane serum level was 3 ppb 20 hours after the application.[913] Because of an inability to obtain a definite dose from this study, no comparison to the HRA estimated route-specific doses could be made.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the NIH-cited benchmark dose discussed above will be used for HRA comparison under the assumption of equivalent absorption in oral and inhalation routes.
B. Subchronic Health Effects
1. Oral Exposure
Effects from the ingestion of lindane can include nausea, vomiting, and such neurotoxic effects as grand mal seizures, visual hallucinations, movement disorders, and coma. Subchronic oral health effects tend to produce much the same response as subchronic dermal health effects.
The NIH-cited human study discussed in the acute oral section above was conducted over a 14-day period. Because this time frame borders between acute and subchronic definitions, this same benchmark dose will be used for acute and subchronic comparisons to the HRA estimated route-specific doses. The assumptions stated in relation to that study remain the same throughout this review.
2. Dermal Exposure
Common neurological effects of subchronic skin exposure to lindane are grand mal seizures, visual hallucinations, movement disorders, restlessness, irritability, confusion, nervousness, dizziness, and coma. Subchronic exposure may also cause defatting dermatitis that is associated with incorrect application of medicinal treatments. According to the National Toxicology Program (NTP), the lowest published toxic dermal dose for humans is 20 mg/kg (during a six-week period).[914] It is uncertain whether this is an absorbed or applied dose and, for this reason, the data from this case study will not be used in comparing HRA route-specific doses with published health effects.
In a case of subchronic lindane poisoning, a 24-year-old woman reported having itchy bumps and visual hallucinations. She also presented with a headache, restlessness, and anxiety. The woman had applied an unknown concentration of lindane over her entire body and left it on 8–12 hours for 24 consecutive days. The lindane blood serum concentration was 7 ppb.[915] Because of an inability to obtain a definite dose from this study, no comparison to the HRA estimated route-specific doses could be made.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the benchmark dose cited by NIH discussed above will be used for HRA comparison under the assumption of equivalent absorption in oral and inhalation routes.
C. Chronic Health Effects
In human chronic exposures to lindane, symptoms include many of the acute exposure health effects listed above, plus degenerative changes in the liver and renal tubules and reduced reproductive capacity in both sexes.[916] Lindane and other organochlorines can be associated with signs and symptoms such as apathy, headache, uncontrolled mood swings, depression, confusion, and irritability in chronic exposures.[917]
One case of chronic lindane poisoning reports of a 45-year old male suffering fleeting episodes of blurred vision that became persistent after 3 months; he then developed occipital headaches and tinnitus. He was diagnosed with chronic intracranial hypertension that resulted from chronic use of lindane. The man had used lindane at least twice a month for 30 years to treat his hunting dogs for fleas and ticks. He used a 20 percent concentrate for spraying and dipping and claimed that he used a mask and proper clothing when applying the pesticide.[918]
In a study of 15 women exposed to wood-preserving chemicals in their homes for an average exposure period of 10 years, researchers found that the mean lindane serum blood levels were 0.085 �g/L (lindane serum levels in unexposed women were 0.043 �g/L). The women exposed to the lindane had subjective complaints and showed an altered neurobehavioral performance through standardized testing.[919]
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Table 159. Lindane, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
| Pesticide | Exp. Level | Type * | Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDdHRA |
PDRDd Literature |
ADDd HRA |
ADDd Literature |
HRA |
Literature |
|||
| L:indane (1% dust) | Low | A |
— |
5.71E–01a,c |
— |
— |
6.53E–05 |
N/A |
6.87E-04 |
5.71E–01a,b |
| Med. | S |
— |
5.71E–01a,c |
— |
— |
1.58E–03 |
N/A |
1.15E-02 |
5.71E–01a,b,c |
|
| High | S |
6.86E–02 |
5.71E–01c,a |
— |
— |
3.81E–02 |
N/A |
2.44E-01 |
5.71E–01a,b,c |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a No-observed-effect-level (NOEL) |
| b Based on acute/subchronic oral data; assumes equivalent absorption. |
| c Benchmark is based on a 14-day study and, therefore, borders between acute and subchronic. |
| d PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
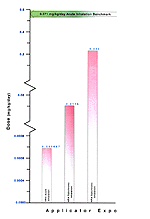
Based on Table 159, it seems the HRA route-specific dose estimates are below the range where human health effects were reported in the literature. To illustrate this, a comparison of doses in a high subchronic oral exposure scenario shows that the dose at which no human health effects were observed is 7.3 times the HRA estimated dose. When comparing high subchronic inhalation exposures, the dose at which no human health effects occur is 1.3 times above the HRA estimated dose. [Figure 26]
E. Uncertainty/Variability of this Comparative Risk Characterization
Because reportable dose response data are lacking, there is significant uncertainty with this risk characterization. As mentioned above, the benchmark dose used in the comparison is based on assumptions of exposure route, absorption equivalency between exposure routes, and uncertainty in the definition of "no ill effects." Further uncertainty arises because of insufficient information about the human test group.
For these reasons, caution should be exercised when drawing conclusions from these data.
F. Risk Communication Summary
Based on published benchmarks, the HRA calculated dose estimates seem to be well below doses at which human health effects occur. It should be noted, however, that this is based on the assumptions communicated throughout this text and a statistically significant comparison is not possible because of a lack of sufficient dose response data.
Figure 26. Representation of Estimated Risk
| First Page | Prev Page | Next Page |