
Contents |
 |
Destinations |
 |
Reference Materials |
 |
Outbreaks |
 |
Traveling with Children |
 |
Special Needs Travelers |
 |
Diseases |
 |
Safe Food and Water |
 |
Vaccinations |
 |
Cruise Ships and Air Travel |
 |
Visitor Survey |
 |
Other Related Sites |
|
 |
U.S. State Department |
|
 |
Division of Quarantine |
|
 |
National Center for Infectious Diseases |
|
 |
The Pan American Health Organization |
|
 |
World Health Organization |
|
 |
Hepatitis,
Viral, Type A
Health Information for International Travel, 1999–2000
Description
Hepatitis A is an enterically transmitted viral disease that causes fever,
malaise, anorexia, nausea, and abdominal discomfort, followed within a few days by
jaundice. The disease ranges in clinical severity from a mild illness lasting 1–2
weeks to a severely disabling disease lasting several months. In developing countries,
hepatitis A virus (HAV) is usually acquired during childhood, most frequently as an
asymptomatic or mild infection. Transmission may occur by direct person-to-person contact;
or from contaminated water, ice, or shellfish harvested from sewage-contaminated water; or
from fruits, vegetables, or other foods that are eaten uncooked, but which may become
contaminated during handling.
Occurrence
Hepatitis A is highly endemic throughout the developing world but of low
endemicity in developed countries such as the United States.
Risk for Travelers
The risk of acquiring HAV infection for U.S. citizens traveling abroad varies
with living conditions, length of stay, and incidence of hepatitis A in the area visited.
Travelers to North America (except for Mexico), developed countries in Europe, Japan,
Australia, and New Zealand are at no greater risk of infection than in the United States.
For travelers to developing countries, risk of infection increases with duration of travel
and is highest in those who live in or visit rural areas, trek in backcountry areas, or
frequently eat or drink in settings of poor sanitation. Nevertheless, many cases of
travel-related hepatitis A occur in travelers to developing countries with
“standard” tourist itineraries, accommodations, and food consumption behaviors.
Hepatitis A vaccine and/or immune globulin (IG) is recommended for all susceptible persons
traveling to or working in countries with intermediate or high endemicity of infection
(see map below).
MAP.
Endemicity patterns (low, intermediate, and high) of hepatitis A virus infection
worldwide. (Note: this map generalizes available data, and patterns may vary within
countries.) |
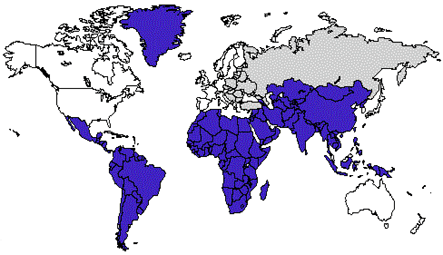 |
| Anti-HAV Prevalence |
 High High
 Intermediate Intermediate
 Low
Low |
NOTE: this map is an updated version of the map that appears in the
1999-2000 edition of Health Information for International Travel. |
| |
|
Vaccine
Two hepatitis A vaccines are currently licensed in the United States: HAVRIX�
(manufactured by SmithKline Beecham) and VAQTA� (manufactured by Merck &
Co., Inc.). Both vaccines are made of inactivated virus adsorbed to aluminum hydroxide as
an adjuvant. HAVRIX� is prepared with 2-phenoxyethanol as a preservative while
VAQTA� is formulated without a preservative. The vaccine should be
administered by intramuscular injection in the deltoid muscle.
Both HAVRIX� and VAQTA� are currently licensed in two
formulations, and the formulation and number of doses vary according to the person’s
age. For HAVRIX�, the schedule for children and adolescents 2–18 years of
age is two 720-EL.U. doses, with the second dose given 6–12 months after the first.
For adults > 18 years of age, the schedule is two 1,440 EL.U. doses with the second
dose given 6–12 months after the first (see Table 2a).ForVAQTA� the
schedule for children and adolescents 2–17 years of age is two 25-U doses given
6–18 months after the first, and for adults > 17 years of age, the schedule is two
50-U doses given 6 months apart (see Table 2b).
Vaccination of children > 2 years of age, adolescents, and adults with the
age-appropriate dose (see Table 2a: Recommended doses of HAVRIX� and Table 2b:
Recommended doses of VAQTA�) is preferred for persons who plan to travel
repeatedly or reside for long periods in high-risk areas. Data on the long-term
persistence of antibody after hepatitis A vaccination are limited because the currently
available vaccines have been under evaluation for only 5–7 years. Estimates of
antibody persistence derived from kinetic models of antibody decline suggest that
protective levels of anti-HAV could persist for at least 20 years. Protection can be
assumed by 4 weeks after receiving the first vaccine dose, although a second dose is
necessary for long-term protection. Because protection may not be complete until 4 weeks
after vaccine administration, persons traveling to high-risk areas < 4 weeks after the
initial dose should also be given IG (0.02 mL/kg) when available, but at a different
injection site.
Travelers < 2 years of age should receive a single dose of IG (0.02 mL/kg) because
neither vaccine is licensed for children in this age group. Travelers who are allergic to
a vaccine component or otherwise elect not to receive vaccine should receive a single dose
of IG (0.02 mL/kg), which provides effective protection against hepatitis A for up to 3
months. For adults and children traveling > 3 months, an IG dose of 0.06 mL/kg should
be given and must be repeated if the duration of travel is > 5 months. See Table 1 for
approximate IG dosages.
Although vaccination of an immune person is not contraindicated and does not increase
the risk of adverse effects, screening for total antibodies to HAV (anti-HAV) before
travel may be useful to determine susceptibility and eliminate unnecessary vaccination or
IG prophylaxis of immune persons. Such serologic screening for susceptibility may be
indicated for adult travelers who are likely to have had prior HAV infection if the cost
of screening (laboratory and office visit) is less than the cost of vaccination or IG
prophylaxis and if testing will not interfere with subsequent receipt of vaccine or IG.
Such persons may include those > 40 years of age and persons born in parts of the world
with intermediate or high endemicity (see map above). Postvaccination testing for
serologic response is not indicated.
Table
1. Immune globulin for protection against viral hepatitis A |
| Length of stay |
Body weight |
Dose volume* |
Comments |
| lb |
kg† |
Short-term
travel
(< 3 mos) |
< 50
50–100
>100 |
< 23
23–45
> 45 |
0.5 mL
1.0 mL
2.0 mL |
Dose volume depends on body weight and length of stay |
Long-term
travel
(3–5 months) |
< 22
< 50
50–100
> 100 |
< 10
< 23
23–45
> 45 |
0.5 mL
1.0 mL
2.5 mL
5.0 mL |
|
| * |
For
intramuscular injection. |
|
| † |
kg =
approximately 2.2 lbs. |
|
|
Table
2a. Recommended doses of HAVRIX�* |
| Group |
Age (years) |
Dose (EL.U.)† |
Volume |
No. of
doses |
Schedule
(months)� |
Children
and
adolescents� |
2–18 |
720 |
0.5 mL |
2 |
0,
6–12 |
| Adults |
>18 |
1,440 |
1.0 mL |
2 |
0,
6–12 |
|
| * |
Hepatitis A vaccine,
inactivated, SmithKline Beecham. |
|
| † |
EL.U = ELISA units. |
|
| � |
0 months represents
timing of the initial dose; subsequent numbers represent months after the initial dose. |
|
| � |
An alternate
formulation and schedule (three doses) are available for children and adolescents,
consisting of 360 EL.U. per 0.5-mL dose at 0, 1, and 6 12 months of age. |
|
|
Table
2b. Recommended doses of VAQTA�* |
| Group |
Age (years) |
Dose (U)† |
Volume |
No. of
doses |
Schedule
(months)� |
Children
and
adolescents |
2–17 |
25 U |
0.5 mL |
2 |
0,
6–18 |
| Adults |
>17 |
50 U |
1.0 mL |
2 |
0,
6–12 |
|
| * |
Hepatitis A vaccine,
inactivated, Merck & Company, Inc. |
|
| † |
Units |
|
| � |
0 months represents
timing of the initial dose; subsequent numbers represent months after the initial dose. |
|
|
Safety
Among adults, the most frequently reported side effects occurring within 3 days
following a dose of HAVRIX� were soreness at the injection site (56%),
headache (14%), and malaise (7%). In clinical studies among children, the most frequently
reported side effects were soreness at the injection site (15%), feeding problems (8%),
headache (4%), and injection-site induration (4%).
Among adults, the most frequent side effects occurring within 5 days following
vaccination with VAQTA� include tenderness (53%), pain (51%), warmth (17.3%)
at the injection site (53%) and headache (16.1%). Among children, the most common side
effects reported were pain (19%), tenderness (17%), and warmth (9%) at the injection site.
Post-licensure reports, without regard to causality, of serious adverse events received
by the vaccine manufacturers have included (but may not be limited to) anaphylaxis,
Guillain-Barr� syndrome, brachial plexus neuropathy, transverse myelitis, multiple
sclerosis, encephalopathy, and erythema multiforme. Most of these events have occurred
among adults, and many have occurred among persons receiving other vaccines concurrently.
Neither vaccine should be administered to persons with a history of hypersensitivity to
alum and HAVRIX� hepatitis A vaccine should not be administered to persons
with a history of hypersensitivity reactions to the preservative 2-phenoxyethanol. Because
the vaccine is inactivated, no special precautions need to be taken for vaccination of
immunocompromised persons.
Any adverse event suspected to be associated with hepatitis A vaccination should be
reported to the Vaccine Adverse Event Reporting System (VAERS). VAERS forms can be
obtained by calling 1-800-822-7967.
Pregnancy
The safety of hepatitis A vaccine for pregnant women has not been determined. However,
because hepatitis A vaccine is produced from inactivated HAV, the theoretical risk to
either the pregnant woman or the developing fetus is thought to be very low. The risk of
vaccination should be weighed against the risk of hepatitis A in women who may be at high
risk for exposure to HAV.
Immune globulin
Immune globulin for intramuscular administration prepared in the United States has few
side effects (primarily soreness at the injection site) and has never been shown to
transmit infectious agents (hepatitis B virus [HBV], hepatitis C virus [HCV] or human
immunodeficiency virus [HIV]). Since December 1994, all IG products commercially available
in the United States must undergo a viral inactivation procedure or be negative for HCV
RNA (ribonucleic acid) before release. Pregnancy is not a contraindication to using immune
globulin.
Preventive Measures
HAV is inactivated by boiling or cooking to 85� C for at least 1 minute.
Cooked foods cannot serve as vehicles for disease unless contaminated after cooking.
Adequate chlorination of water as recommended in the United States will inactivate HAV. In
developing countries, travelers should minimize their risk of hepatitis A and other
enteric diseases by avoiding potentially contaminated water or food. Drinking water (and
beverages with ice) of unknown purity, uncooked shellfish, and uncooked fruits or
vegetables that are not peeled or prepared by the traveler should be avoided.
See the Diseases section for
more information on hepatitis A.
|


