According to the RAND survey, up to 12% of servicemembers used or witnessed the use of fly baits, while the PM interviews indicate a usage rate of at least 43%. In addition to the survey and PM interviews, investigators conducted fly bait interviews from December 1997 through February 1998 to get specific exposure data on fly baits. Fly baits were applied not only by trained applicators, but widely applied by untrained servicemembers.
The exposure assessment for methomyl, 1% crystals, is similar in most respects to that done for azamethiphos, 1% crystals. The one difference between the two exposure assessments is the consideration of exposure to methomyl vapor in tanks. Investigators assumed vapor release was potentially consequential for methomyl due to its higher vapor pressure, whereas vapor release was not a consequential factor for azamethiphos. There were a few reports of servicemembers using fly baits in tanks, and many servicemembers spent substantial amounts of time in tanks and other vehicles. According to the survey (Table 12), 10% of Army servicemembers slept in vehicles, and EPA[189] recommended considering exposure to methomyl vapor released from fly baits into tanks.
Table 39 presents the common assumptions for application exposure. The low exposure level represents application personnel who operated under favorable conditions to minimize exposure; that is, servicemembers were located in permanent facilities in urban areas, fly populations were minimal, gloves were used, and personal hygiene was good. The consequential exposure routes were dermal contact and inhalation.
Table 39. Fly baits, common assumptions for applicationa
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale |
||
| Low | Medium |
High |
||||
| UE | mg/lb a.i. |
Unit dermal exposure for dispersal | 71 |
71 |
71 |
1998 PHED Guide[190] |
| UIE | mg/lb a.i. |
Unit inhalation exposure for dispersal | 0.47 |
0.47 |
0.47 |
1998 PHED Guide[191] |
| WF | lb/d |
Weight of formulation handled | 1 |
2 |
4 |
See note;b product label[192] |
| CS | mg/kg |
Concentration of a.i. in the formulation | 10,000 |
10,000 |
10,000 |
1% = 10,000 mg/kg |
| WA | lb a.i./d |
Weight of a.i. handled | 0.02 |
0.04 |
0.08 |
WF x 0.01 |
| CF | kg/mg |
Unit conversion factor | 1E-06 |
1E-06 |
1E-06 |
Standard |
| Events | d-1 |
Dermal exposure events | 1 |
1 |
1 |
See notec |
| SA | cm2 |
Skin surface area available for contact | 210 |
210 |
210 |
EPA[193] |
| IR | mg/d |
Ingestion rate | -- |
-- |
50 |
EPA;[194] EPA[195] |
| AF | mg/cm2 |
Dust-to-skin adherence factor | 0.047 |
0.047 |
0.47 |
EPA;[196] PHED Guide[197] |
| a) | A dash ("--") indicates that the item is not applicable. |
| b) | There is no information available on how much formulation was actually applied by an applicator on a given day. Given the application rate on the label, and the size of the container, combined with the general in formation from fly bait interviews and PM interviews, a range of 1 to 4 lb per day per applicator was selected. |
| c) | Assumption of 1 exposure event per day is reasonable given that dermal transfer of a.i. from particle-bound residues is likely to have been extremely slow. |
The medium exposure level represents servicemembers who operated under fair conditions; that is, reasonably clean, well constructed temporary facilities in semi-rural areas, where fly populations were moderate, gloves were used, and personal hygiene was good. The consequential exposure routes were dermal contact and inhalation.
The high exposure level represents application personnel who operated under unfavorable conditions; that is, servicemembers were located in temporary facilities in rural locations, fly populations were very high, gloves were frequently not used, and personal hygiene was poor. The consequential exposure routes were incidental ingestion, dermal contact, and inhalation.
Investigators handled dermal exposure of applicators by combining two inputs: the unit dermal exposure (UE) plus the exposure of one hand, gloved or not, as appropriate. The factor "events" represents the number of exposure events occurring per day where the skin surface area available for contact (SA) is covered by formulation dust, and all the active ingredient present in dust contacting skin is available for absorption. The investigators selected the value of 1 for events. In fact, the dermal transfer of active ingredient from particle-bound pesticide active ingredient residues is likely to be extremely slow. EPA’s Office of Pesticide Programs specifically questioned the bioavailability of dermal exposure to particle-bound pesticide active ingredient residues.[198] Investigators selected the dust-to-skin adherence factor (AF) of 0.47 mg/cm2 for the high-exposure group because it is a high-end published value. Investigators reduced the AF by a factor of 10 for the low and medium exposure groups based on assumed glove usage.
b. Azamethiphos
Twenty-seven percent of the PM exposure interviews cited use of azamethiphos, 1% crystals. Table 40 presents the specific assumptions for azamethiphos crystals.
Table 40. Azamethiphos assumptions for applicationa
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale |
||
| Low | Medium |
High |
||||
| ET | h/d |
Exposure time | 1 |
4 |
8 |
PM interviews (Table 13); see noteb |
| EF | d/mo |
Exposure frequency | 4 |
22 |
30 |
PM interviews (Table 13) |
| ED | mo |
Exposure duration | 3 |
5 |
9 |
PM interviews (Table 13) |
| ABS | -- |
Dermal absorption factor | 0.27 |
0.27 |
0.27 |
Ciba-Geigy[199] |
| a) | A dash ("--") indicates that the item is not applicable. |
| b) | The low value is the 10th percentile from the PM exposure interviews. It is unlikely that personnel spent more than 8 hours per day applying fly baits. |
c. Methomyl
Forty-three percent of the PM exposure interviews cited use of methomyl, 1% crystals. Table 41 presents the specific assumptions for methomyl crystals.
Table 41. Methomyl assumptions for applicationa
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale |
||
| Low | Medium |
High |
||||
| ET | h/d |
Exposure time | 1 |
4 |
8 |
PM interviews (Table 13); see noteb |
| EF | d/mo |
Exposure frequency | 1 |
18 |
30 |
PM interviews (Table 13) |
| ED | mo |
Exposure duration | 1 |
4 |
8 |
PM interviews (Table 13) |
| ABS | -- |
Dermal absorption factor | 0.5 |
0.5 |
0.5 |
Hayes et al.[200] |
| a) | A dash ("--") indicates that the item is not applicable. |
| b) | The low value is the 10th percentile from the PM exposure interviews. It is unlikely that personnel spent more than 8 hours per day applying fly baits. |
d. Fly Bait Doses — Application
Table 42 presents doses potentially resulting from exposure during application of fly baits. There are four types of doses presented for the evaluation of noncarcinogenic effects: PDRO, PDRD, ADD, and PDRI. The manufacturer has not associated azamethiphos with carcinogenic activity, and EPA has not associated methomyl with carcinogenic activity (see Section B.4, Toxicity Assessment), so investigators did not calculate LADDs.
Table 42. Fly baits, dose rates – application, for evaluation of noncarcinogenic effectsa
Formulation |
Exposure |
ABS |
PDRO |
PDRD |
ADD |
PDRI |
||||||
| Azamethiphos, 1% crystals |
Low | 0.27 |
-- |
1.16E-02 |
3.12E-03 |
6.71E-05 |
||||||
| Medium | 0.27 |
-- |
2.17E-02 |
5.86E-03 |
1.34E-04 |
|||||||
| High | 0.27 |
7.14E-03 |
5.47E-02 |
1.48E-02 |
2.69E-04 |
|||||||
| Methomyl, 1% crystals |
Low | 0.5 |
-- |
1.16E-02 |
5.78E-03 |
6.71E-05 |
||||||
| Medium | 0.5 |
-- |
2.17E-02 |
1.08E-02 |
1.34E-04 |
|||||||
| High | 0.5 |
7.14E-03 |
5.47E-02 |
2.73E-02 |
2.69E-04 |
|||||||
|
||||||||||||
| a) | ABS = dermal absorption factor. |
| PDRO = potential dose rate for ingestion. | |
| PDRD = potential dose rate for dermal contact. | |
| ADD = absorbed dermal dose. | |
| PDRI = potential dose rate for inhalation. | |
| A dash ("--") indicates that the item is not applicable. | |
| IR = ingestion rate. | |
| CS = concentration of a.i. in formulation. | |
| CF = unit conversion factor. | |
| BW = body weight. | |
| UE = unit dermal exposure. | |
| WA = weight of a.i. handled. | |
| SA = skin surface area available for contact. | |
| AF = adherence factor. | |
| Events = dermal exposure events. | |
| UIE = unit inhalation exposure. | |
| b) | Formula 2 adds dermal exposure to hand to other dermal exposure calculated per the PHED Guide. PHED does not provide values for hands in this scenario. |
a. Common Elements
Investigators did not evaluate post-application exposure for the low-exposure group because they presumed it was inconsequential. The following factors would have served to keep exposure levels very low: low levels of pests, low levels of fly bait usage, use mainly outside, use per label directions, and good hygienic practices.
Investigators quantitatively evaluated neither post-application dermal exposure to fly baits, nor inhalation of fly bait particulates, although both presumably occurred. EPA’s Office of Pesticide Programs questions the bioavailability of dermal exposure to particle-bound pesticide active ingredient residues and would not normally address the dermal exposure scenario in the present circumstance.[201] Similarly, OPP questions the bioavailability of active ingredient inhaled in particulates, as most such particles would be swallowed as a result of mucocilliary action, in particular since the majority of particles would probably have exceeded 10 microns in diameter. We did evaluate exposure to methomyl vapor in tanks, but did not for azamethiphos, for reasons described below.
Investigators assumed substantial use of fly baits inside tents and other structures at the medium-exposure level and, especially, at the high-exposure level. Both the PM exposure interviews (Table 13) and the fly bait interviews (Table 16) support this approach.
Investigators conducted exposure modeling to estimate the exposure point concentrations (EPCs) for post-application incidental ingestion. Variations of the soil/dust model and assumptions used to derive the EPCs for fly baits are presented in Tables 43 through 46. Investigators developed the model expressly to estimate exposures due to fly baits. The primary assumption of the model is that the net amount of active fly bait material available in soil and dust on any given day is equal to the amount released in a single day, as defined in the model; that is, it is assumed that steady-state conditions exist. Under the actual conditions of usage there were certainly gains and losses of active material each day; however, the daily average that was actually available is unknown. The differences in usage between the fly bait formulations was minimal. Thus, all post-application assumptions are listed in Table 47.
b. Azamethiphos
Ingestion is the only consequential post-application exposure route for medium and high exposure levels for azamethiphos, 1% crystals. The release of azamethiphos vapor directly to the air constitutes an inconsequential exposure pathway, given that the vapor pressure of azamethiphos at 20oC is 3.7 x 10-8 mm Hg.[202] Thus, investigators did not conduct air modeling for azamethiphos vapor. OPP is in agreement with this assessment.[203]
Table 43. Estimated fly bait concentration in soil/dust, mess tent, GP large
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 44. Estimated fly bait concentration in soil/dust, mess building, temporary
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 45. Estimated fly bait concentration in soil/dust, latrine, "three-holer"
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 46. Estimated fly bait concentration in soil/dust, M-1A Tank
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 47. Fly bait assumptions for post applicationa
| Factor | Units | Definition/Explanation | Assumptions by Level |
Source/Rationale |
||
| Low | Medium |
High |
||||
| CS | mg/kg |
Concentration of a.i. in indoor soil/dust | -- |
6 |
226 |
Tables 43 and 46 |
| CF | kg/mg |
Unit conversion factor | -- |
1E-06 |
1E-06 |
Standard |
| CA | mg/m3 |
Concentration of methomyl in air inside tank | -- |
-- |
0.19 |
Air modeling, 8-hour average |
| IR | mg/d |
Ingestion rate | -- |
50 |
480 |
EPA;[218,219] |
| ET | h/d |
Exposure time to air inside tank | -- |
-- |
8 |
See noteb |
| EF | d/mo |
Exposure frequency | -- |
30 |
30 |
Survey (Table 13); Fly bait interviews (Table 16) |
| ED | mo |
Exposure duration | -- |
5 |
8 |
Fly bait interviews (Table 16) |
| a) | A dash ("--") indicates that the item is not applicable. |
| b) | It is unlikely troops spent more than 8 hours per day in a tank under most circumstances. |
c. Methomyl
Ingestion is the only consequential post-application exposure route for the medium exposure level for methomyl, 1% crystals. Ingestion, and inhalation of vapor are consequential at the high exposure level. OPP suggested that inhalation of methomyl vapor released from fly baits would only be of potential concern under one circumstance: release of vapor into a confined space such as a tank.[220] Investigators conducted air modeling to evaluate the latter possibility, as described in the following subsection.
d. Air Modeling for Methomyl
Investigators conducted air modeling to estimate potential exposure to servicemembers within tanks to methomyl vapors released from fly bait crystals scattered inside tanks. As recommended by EPA, investigators calculated average emission rates from applied methomyl crystals using an empirical relationship for evaporation time based on the vapor pressure and molecular weight of the active ingredient by the method of Chinn.[221] This relationship is based on the evaporation of pure substances under artificial conditions and probably overestimates the emissions from fly bait crystals.
In this approach, one first calculates the Chinn evaporation time using the following formula:
te = 145/[(mw)(vp)]0.9546
where,
te = Chinn evaporation time (h)
mw = molecular weight of active ingredient (unitless)
vp = vapor pressure of active ingredient (mm Hg)
Next, one calculates a long-term emission rate using the following formula:
E = mass/d
where,
E = emission rate (mg/h)
mass = mass of active ingredient (mg)
d = day
Investigators calculated the mass of active ingredient applied inside the tanks (5.3 g) from the product of the mass of fly bait applied in the tanks (530 g) and the fractional component of the active ingredient in the fly bait (1%).
One obtains an upper bound value for methomyl in tanks by calculating an air saturation concentration. The saturation concentration is provided by the following expression:
CAsat = (vp/760)(mw)(106)/[(R)(T)]
where,
vp = vapor pressure (mm Hg)
mw = molecular weight (g/mole)
R = 0.0821 liter atmosphere/mole oK, the universal gas constant
T = temperature of air (oK)
Investigators assumed a temperature of 293o K (20o C) for the calculation of the saturation concentration. The sensitivity of saturation concentration to temperature is relatively low over the range of temperatures that might be expected inside a tank, since the saturation concentration is inversely proportional to the absolute temperature.
The saturation air concentration should be regarded as an extreme upper bound estimate. It represents the concentration that might occur after a sufficient time in the absence of adequate ventilation. However, it almost certainly overestimates concentrations that would be expected to occur during normal usage inside a tank treated with methomyl crystals.
One can estimate more realistic concentrations by using a simple box model approach. One can develop the box model equation from the same mass balance considerations described for permethrin. Likewise the differential equation is the same, although the inputs vary somewhat:
V(dC/dt) = E + CaIV – CIV – KCV
where,
C = concentration (mg/m3)
Ca = ambient (outdoor) concentration (mg/m3)
E = emission rate (mg/h)
I = air changes per hour in room
V = room volume (m3)
t = time (h)
K = decay rate (h-1)
This equation has the following general solution:
C = [1/(I+K)][(E/V) + (Ca)(I)][1 – exp{-(I+K)(t)}] + Co exp{-(I+K)(t)}
where,
Co = initial concentration in room (mg/m3)
Investigators assumed: 1) active ingredients to be nonreactive (K=0), and 2) contributions from outdoors to be negligible (Ca = 0); the rationale is explained under permethrin.
With these assumptions, the equation for concentration within the room simplifies to:
C = [E/(I)(V)][1 – exp{-(I)(t)}] + Co exp{-(I)(t)}
For an initial concentration of zero (Co = 0), this simplifies to:
C = [E/(I)(V)][1 – exp{-(I)(t)}]
This equation yields estimated concentrations that asymptotically approach an equilibrium concentration given by:
C = E/[(I)(V)]
The time in hours (tf) required to reach a given fraction (f) of the equilibrium concentration is:
tf = -[ln(1-f)]/I
The exposure scenarios of interest involve servicemembers spending an extended period of time (for example, 8 hours) inside a tank with little or no ventilation. This exposure scenario should be considered hypothetical based on the following considerations. Tanks have three hatches, two on top of the turret and one in the forward hull above the driver. Anecdotal evidence indicates that these hatches were generally kept open in the Gulf and that the time spent "buttoned up" (i.e., with hatches closed) was minimal. In addition, the tanks used in the Gulf War had ventilation systems that would likely have been used if all hatches were closed for an extended period of time.
The M1A1 tank has an environmental control/nuclear, biological and chemical (EC/NBC) system that provides filtered air to servicemembers via masks. The air exchange rate associated with this system is fairly high (0.092 m3/sec), corresponding to an air turnover rate on the order of about 50 changes per hour. The M1 tank has a blower in the turret that is used after firing and perhaps at other times as well. The air exchange rate associated with the blower (0.014 m3/sec) is on the order of 8 changes per hour.
However, other anecdotal evidence suggests that the tank ventilation systems were infrequently used due to the noise they generated. As mentioned before, the hatches in the tanks were generally left open. The ambient winds characteristic of the area (or the relative wind experienced when the tank is in motion) would be expected to induce some air turnover in the tanks when the hatches were left open. It is difficult to estimate ventilation rates that would be generated by the wind or by motion of the tank when the hatches are open. Nonetheless, calculations for some assumed low ventilation rates indicate that concentrations inside the tank should not approach the saturation concentration presented earlier.
Interior air volumes fall in the range of 5.7 - 7.3 m3 for M1A1 tanks and in the range of 5.7 – 6.8 m3 for M1 tanks. Investigators selected the low end of these ranges for the calculations in order to estimate the largest concentrations.
Air exchange rates of 0.5, 0.25, and 0.125 air changes per hour (corresponding to 4, 2, and 1 air changes per 8-hour period, respectively) were selected to represent low, medium, and high exposure scenarios over the 8-hour period. Investigators calculated equilibrium concentrations for each ventilation rate and, in the case of the lowest air exchange rate considered, capped at the saturation concentration (about 85% of the calculated equilibrium concentration).
The estimated time needed to reach the saturation concentration for the high exposure scenario (about 15.5 hours) exceeds the duration of the exposure scenario, so estimated average concentrations over the 8-hour period will be less than the calculated saturation concentrations. In addition, estimated average concentrations over the 8-hour period should be less than equilibrium concentrations due to the time needed for concentrations to build up to near the equilibrium levels.
One can obtain average concentrations over the time period from t = 0 to t = T by integrating the concentration equation C = [E/(I)(V)][1 – exp{-(I)(t)}] over the period and dividing by the duration of the period, yielding:
Cavg = [E/(I)(V)(T)][T + (1/I)(exp{-(I)(T)}-1)]
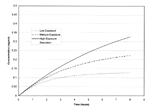
Table 48 summarizes the calculated 8-hour average methomyl concentrations for the modeled exposure scenarios. Figure 11 is a time plot of estimated methomyl concentrations in the tank versus time for each of the three defined exposure scenarios. The estimated concentrations are well below the saturation concentration (0.44 mg/m3) even for the low assumed ventilation rates.
Table 48. Methomyl air modeling results
Case |
Scenario |
8-hour Average Concentration(mg/m3) |
1 |
Low exposure |
0.0977 |
2 |
Medium exposure |
0.147 |
3 |
High exposure |
0.190 |
Figure 11. Time plot of methomyl concentrations in tanks
A table summarizing the calculation of 8-hour average methomyl concentrations for the three defined low ventilation scenarios is presented in Table 49. The table also presents the saturation concentration and the estimated ending and equilibrium concentrations for each scenario.
Table 49. Calculation of concentrations inside a tank for methomyl, 1% crystals
Exposure Scenario |
High |
Medium |
Low |
| Molecular Weight a.i. (g/mole) | 162.2 |
162.2 |
162.2 |
| Vapor pressure a.i. (mm Hg) | 5.00E-05 |
5.00E-05 |
5.00E-05 |
| Mass of fly bait (g) | 530 |
530 |
530 |
| Fraction of methomyl in fly bait | 0.01 |
0.01 |
0.01 |
| Mass of methomyl (mg) | 5300 |
5300 |
5300 |
| Chinn evaporation time (hours) | 1.44E+04 |
1.44E+04 |
1.44E+04 |
| Chinn evaporation rate (mg/h) | 3.69E-01 |
3.69E-01 |
3.69E-01 |
| Saturation concentration (mg/m3) | 4.44E-01 |
4.44E-01 |
4.44E-01 |
| Volume inside tank (m3) | 5.7 |
5.7 |
5.7 |
| Air changes per hour | 0.125 |
0.25 |
0.5 |
| Duration of exposure (hours) | 8 |
8 |
8 |
| Air changes per scenario duration | 1 |
2 |
4 |
| Equilibrium concentration (mg/m3) | 4.44E-01 |
2.59E-01 |
1.29E-01 |
| Concentration at end of period (mg/m3) | 3.27E-01 |
2.24E-01 |
1.27E-01 |
| Average concentration (mg/m3) | 1.90E-01 |
1.47E-01 |
9.77E-02 |
e. Fly Bait Doses — Post Application
Table 50 presents doses potentially resulting from post-application exposure to fly baits. There are two types of doses presented for the evaluation of noncarcinogenic effects: PDRO and PDRI. The manufacturer has not associated azamethiphos with carcinogenic activity, and EPA has not associated methomyl with carcinogenic activity (see Section B.4, Toxicity Assessment), so investigators did not calculate LADDs.
Table 50. Fly baits, dose rates – post application, for evaluation of noncarcinogenic effectsa
Formulation |
Exposure Group |
ABS |
PDRO |
PDRD |
ADD |
PDRI |
||||
| Azamethiphos, 1% crystals |
Low | 0.03 |
-- |
-- |
-- |
-- |
||||
| Medium | 0.03 |
4.29E-06 |
-- |
-- |
-- |
|||||
| High | 0.03 |
1.55E-03 |
-- |
-- |
-- |
|||||
| Methomyl, 1% crystals |
Low | 0.04 |
-- |
-- |
-- |
-- |
||||
| Medium | 0.04 |
4.29E-06 |
-- |
-- |
-- |
|||||
| High | 0.04 |
1.55E-03 |
-- |
-- |
3.47E-02 |
|||||
|
||||||||||
| a) | ABS = dermal absorption factor. |
| PDRO = potential dose rate for ingestion. | |
| PDRD = potential dose rate for dermal contact. | |
| ADD = absorbed dermal dose. | |
| PDRI = potential dose rate for inhalation. | |
| A dash ("--") indicates that the item is not applicable. | |
| SA = surface area available for dermal contact. | |
| BW = body weight. | |
| CA = a.i. concentration in air from modeling. | |
| IRA = inhalation rate. | |
| ET = exposure time. |
| First Page | Prev Page | Next Page |