TAB C-8 – Human Health Benchmarks
I. OVERVIEW
As stated in the health risk assessment (HRA; Part B), it is possible to conduct toxicity assessment and risk characterization using a "toxicological approach," and/or an "epidemiological approach." Part B focused mainly on the toxicological approach; this section focuses on the epidemiological approach. This section is a comparison of human health effects found in the literature with dose estimates from the exposure assessment (EA) portion of the HRA. It supplements means to other sections of the HRA in judging the potential significance of the pesticide exposures estimated for Gulf War veterans. This section can also be called a "human toxicological approach," because it relies on a variety of human toxicology studies described later. Human toxicological data can be of variable quality because scientific gaps exist in exposure and effect information. The goal of this section is to answer the following core questions using available data from human studies:
| 1) | At what exposure levels, route, or combined routes do measurable reported physiological changes, signs, or symptoms occur in human beings from the active ingredients found in the pesticides of potential concern (POPCs)? |
|
| 2) | How do the estimates in the HRA directly compare with human health effect benchmarks identified from scientific literature? |
|
This section provides the results of a literature search on the toxicological benchmarks for human health effects from exposure to the 12 pesticide active ingredients examined in the HRA. Literature values are then compared directly with exposure levels estimated in the HRA (see Part B) to determine the likelihood of adverse health effects.
Literature research criteria were limited to human health effects data regarding exposure to pesticide active ingredients. Unless human experience was part of the health effect or toxicity endpoint decision, the literature search was purposely exclusive of any animal or plant data. Qualitative data regarding human metabolism and health effects were included in the summaries if deemed useful to answering the core question about human toxicity benchmarks.
Human dose-response data for exposure to pesticide active ingredients came from a variety of human toxicology studies including epidemiological, occupational, medical incident and controlled laboratory settings. Such data were the prime sources sought in the scientific literature for all 12 pesticide active ingredients of interest in this study. In some instances doses are estimated when plausible and when actual measured doses for humans were unavailable. If doses were estimated for human health effects found in the literature, the estimate assumptions were reported.
Human health effects data were sought for oral, dermal, inhalation and multiple routes for acute, subchronic and chronic exposures. All human health effect doses found in the literature, or their absence, for all exposure routes and scenarios were described in this report and its accompanying tables. All supporting references for health effects were cited.
Estimated Gulf War Veteran exposures, as summarized in the HRA, were compared directly to the documented human health effect benchmarks (HHEB). Uncertainty and safety factors applied by various organizations, such as USEPA were not applied in this report. Elsewhere in the HRA non-cancer and cancer reference doses based upon the toxicological approach, incorporating animal data and a variety of intraspecies and interspecies uncertainty factors, are compared with HRA dose estimates. To assist with comparing HHEB with the HRA exposure estimates, direct comparisons without any uncertainty factors were employed.
The scientific literature search, while comprehensive, was by no means exhaustive. It encompasses a 2-month search process covering a significant portion of the available and published scientific literature for human health effects. The possibility exists for proprietary research, unpublished research, manufacturer human experience data, or information about human health effects from data sources unavailable or uncovered during the course of the study to provide more complete information on human health effects from exposure to pesticide active ingredients.
II. DEET (N, N-Diethyl-m-Toluamide)A. Acute Health Effects
1. Oral Exposure
In humans, a study was conducted on a number of cases of toxic reactions after ingestion of insect repellents containing DEET. The first case study involved a 14-year-old Canadian girl who had ingested the contents of a 50-mL bottle of insect repellent (95 percent DEET, 5 percent related toluamides). Within 30 minutes of being taken into custody, she was unconscious. Upon arrival at the hospital, mechanical ventilation was instituted. Approximately 90 minutes later, she had a generalized seizure that was successfully treated. Tremors persisted for the next 2 hours. Extensive clinical investigations uncovered no other etiology for her illness. At discharge 10 days after admission, she was physically and neurologically normal.
The second case study involved a 1-year-old girl whose 3�-year-old brother fed her approximately 25 mL of a 50-mL bottle of insect repellent (47.5 percent DEET, 2.5 percent toluamides). While en route to the hospital, the child had a brief (less than 1 minute) seizure. During her 40-minute stay in the emergency room, the child experienced four similar clonic spells. Extensive clinical investigations uncovered no other etiology for her illness. The following 20 hours in the hospital were uneventful, after which the child was discharged to go home.
Within the overall study (two of which have been detailed), each human patient had ingested large amounts of concentrated (47.5 percent–95 percent) insect repellent product. Their common symptoms and signs were coma, seizures, and hypotension, all occurring within 1 hour of ingestion of the insect repellent product. Two of the subjects died. The study concluded that the "ingestion of DEET can produce severe toxic reactions of rapid onset that may be fatal in some instances."[771]
One patient was documented as having ingested 8 oz of DEET in a successful suicide. Symptoms included cardiorespiratory arrest and status epilepticus.[772] For an acute oral exposure, this ingestion indicates an exposure level of 2400 mg/kg/d.
In other cases, urticarial skin reactions or gastrointestinal problems following ingestion were experienced. Also, neurotoxicity was reported in young children after prolonged dermal use or after appreciable ingestion, which was manifested as an encephalopathy with anxiety, behavioral changes, abnormal movements, lethargy, ataxia, mental confusion, seizures, and coma. DEET was detected in the blood of human volunteers within 2 hours of application on the volar surface of the foreman, and there was little evaporation from the skin. In one study, 48 percent of the dermally applied dose was absorbed within 6 hours. DEET is excreted primarily in the urine, and levels of DEET in body fat and the sciatic nerve were found to be higher than levels in plasma.[773]
2. Dermal Exposure
Between 1989 and 1995, the New York State Department of Health was notified of three cases of seizure related to dermal application of DEET. Two of the cases involved children: a 3-year-old girl and a 2-year-old boy. Both of the children recovered, although the girl reportedly went into cardiac arrest and was revived with CPR. The third case involved an adult male who applied the product twice, had a seizure, and died from choking on the food he was eating at the time of the seizure. Two annual reports were submitted by the DEET Joint Venture/Chemical Specialties Manufacturers Association covering all cases reported in 1995 and 1996. In 1995 and 1996, 32 cases mentioned some type of seizure activity that could not be ascribed to another likely cause based on the data initially collected. A number of the cases reported in 1995 and 1996 are still being followed to obtain medical records and complete the supplemental questionnaire forms. When the review is completed, it might be revealed that health effects occurred from causes other than DEET exposure.[774]
In a human dermal absorption study by the US Environmental Protection Agency, radiolabeled DEET in either 15 percent ethanol solution (12 mg; 36 �Ci) or undiluted (15 mg; 37 �Ci) was dermally applied to two groups of healthy human volunteers (6 males/group; ages ranging from 20–29 years). The test material was applied on an area of 4 � 6 cm� of the forearm for 8 hours. The results showed that a small percentage of dermally applied DEET was absorbed. The rate and amount of absorption were greater in the individuals who were treated with the 15 percent DEET solution. The level of radioactivity in the plasma declined rapidly after cessation of exposure. No human health effects data were documented from this study.[775]
A comparative study of dermal absorption on human tissue was conducted by the Health Canada Environmental Health Centre using three commonly used commercial insect repellent formulations. The three formulations — Off!�, Deep Woods�, and Muskol� — contained 14 percent, 24 percent, and 95 percent DEET, respectively. The total percentage of dermal absorption for the three formulations was 48 percent, 36 percent, and 17 percent for human skin, respectively. The study indicated that significant total cumulative quantities of DEET might be absorbed through the skin. Using the 17 percent absorption rate for Muskol�, 2.4 g of DEET would be absorbed by the 66-kg human volunteer who applied 15 mL of Muskol�. This represents an internal dose of 36 mg/kg. Two doses over an 8-hour workday would be a total dose of 72 mg/kg, which amounts to a total dose of 5.0 g for the average 70-kg person. The study indicated that none of the exposure levels presented a hazard to human health.[776]
Another study profiled the cumulative amount of DEET permeated across the skin from commercial mosquito repellents. DEET was found to continuously permeate (from the control and the four products containing DEET) through the human skin throughout a 36-hour period in significant amounts (0.5–1.5 mg/cm�). The cumulative amount of DEET permeated was proportional to the percentage of DEET found in the mosquito repellent. The study concluded that the permeation of DEET continues at high steady-state flux for 36 hours, and may continue to be released into the circulatory system hours after removal of the mosquito repellent from the skin surface. According to the study, none of the indicated levels of exposure presented a human health hazard.[777]
Reports of adverse events are rare, but most occur in children. The American Academy of Pediatrics (AAP) recommends application of only diluted DEET (<10 percent) to children. One study documented a survey of 71 poison control centers (PCCs) participating in the American Association of Poison Control Centers’ National Data Collection System for reports of adverse reactions to DEET between 1985 and 1989. The PCCs covered a wide area of the United States with a population of more than 180 million people. The survey indicated that there were 9,086 human exposures involving DEET-containing insect repellents reported to PCCs between 1985 and 1989. Of those exposed, 98.9 percent experienced either no effect or had short-lived symptoms involving mild irritation to the skin or mucous membranes. A total of 66 patients had symptoms classified as moderate (i.e., more pronounced or more prolonged than the minor effects), but all symptoms resolved. Five patients experienced more dramatic effects after being exposed to products containing 11–50 percent DEET. Two of the five patients experienced eye irritation, which was treated at home. One patient, a 17-year-old male who had saturated his clothing with 17.9 percent DEET, was ataxic and might have had a seizure. A 33-year-old male reported diminished sensation and hypotension 1 week after using a DEET product. Both individuals recovered fully. The study concluded that given the extensive use of DEET and lack of human clinical literature, the risk of adverse effects from the label-directed use of DEET-containing repellents is low.[778]
A 20-year-old soldier sought medical treatment when he experienced a persistent burning sensation and skin eruption after he had applied an insect repellent containing 33 percent DEET about 8 hours before the skin eruption had appeared. A diagnosis of irritant contact dermatitis was made based on the anamnestic and clinical data.[779]
DEET was detected in the blood of human volunteers within 2 hours of application on the volar surface of the forearm, and there was little evaporation from the skin. In one study, 48 percent of the dermally applied dose was absorbed within 6 hours.[780]
According to the USEPA, since 1960 there have been 14 cases of seizure (including four deaths) potentially related to DEET exposure for which other more likely causes have not been identified. An additional 32 cases of seizure reported to the national DEET registry are currently under review. Thus, the final number of potential DEET-associated seizures since 1960 will fall between 14 and 46 cases. This range is subject to both over- and underreporting. Seizure coinciding with DEET use can be expected, given an estimated 15,000–20,000 afebrile (occurring without a fever) seizures in children ages 0–19 years estimated annually and an estimated 17 million children using DEET as often as 10 times a year. On the other hand, physicians may fail to check for history of DEET use or fail to report cases of seizure subsequent to DEET use. As noted in the Morbidity and Mortality Weekly Report editorial on the five cases in 1989, "Anecdotal reports of seizures are difficult to interpret. Taking all the cases together, it does appear that some of the cases are likely related to DEET toxicity, though it is not possible with certainty to say which ones."[781]
In 1989, the EPA notified the Centers for Disease Control and Prevention (CDC) and the Poison Control Center (PCC) through a physicians’ advisory of their concern for these health effects and asked them to report any new cases. The EPA also urged the manufacturer to undertake a review of PCC records. Neither the manufacturer’s review of more than 9,000 DEET exposures nor the EPA’s physicians’ advisory revealed any new cases of seizure that could be substantiated with medical records. Given only 14–32 cases since 1960 (the first case was reported in 1961) and 50–80 million people using DEET each year, the observed incidence of recognized seizures is about 1 per 100 million users.[782]
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
B. Subchronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
1. Oral Exposure
Exposure to DEET occurs in the short to intermediate term; chronic exposure is not expected.[783] Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Data for estimating exposures from conventional consumer use practices are limited and insufficient. There are only a few reported estimates of exposure to occupational groups. Estimates of exposure over 6 months extrapolated from these limited data are: the upper 1 percent of the general population (estimated from a survey of 71 employees of 1 company) were exposed to a 15 percent concentration of DEET at a level of >1.65 g/dose for an exposure quantity of >214 g. Military personnel were exposed to a 75 percent concentration of DEET at a level of >8.25 g/dose for an exposure quantity of >1071 g. The upper 5 percent of Everglades Park Service employees surveyed were exposed to a 15 percent–75 percent concentration of DEET at a level of >2 kg/7 months (1 mL of a 75 percent formulation, 60/year) for an exposure quantity of >1710 g. For chronic dermal exposure, this indicates an exposure level of 136 mg/kg/d. National Park Service workers in the Florida Everglades, who rely heavily on DEET in summer months, have reported episodes of confusion and an abnormal sensation of decreased sweating while using DEET. Response to a neurobehavioral questionnaire by 143 of these workers indicated that there was a significant increase in the prevalence of certain neurological signs and symptoms, specifically muscle cramping, insomnia, irritability, and depression, among the workers who had an estimated dermal exposure to 4.25 g or more of DEET in an average week. Skin rash or blisters and difficulty in starting or stopping the urinary stream were significantly higher in this group of workers.[784]
Though the empirical testing does not demonstrate significant human toxicity to DEET, there are a few reports of individuals experiencing adverse effects after using a DEET product. In the past 35 years, 14 individuals reported suffering seizures after being exposed to DEET: 12 were children. Among these 14 incidents are 5 incidents that were reported to the New York State Department of Health in 1989. The USEPA has analyzed these incidents and, at this time, cannot conclude that these seizures are or are not directly related to DEET exposure. In summary, the EPA concludes that, based on the currently available data, the use of DEET as an insect repellent does not pose a significant health risk to the US population in general for the following reasons: (1) DEET is not believed to be acutely toxic or carcinogenic, or significantly developmentally toxic or mutagenic at the doses tested; and (2) the available data do not support a direct link between exposure to DEET and reported seizure incidences (14 cases). The EPA concludes that DEET insect repellents will generally not cause unreasonable risks to humans or the environment.[785]
3. Inhalation Exposure
Because exposure to DEET occurs in the short to intermediate term, chronic exposure is not expected.[786] Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Application hazards for DEET were not modeled in the HRA Risk Characterization Table (Table 139) below so data are not compared.
Table 139. DEET, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
| Pesticide | Exp. Level |
Type * |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRD b HRA |
PDRD b Literature |
ADD b HRA |
Literature |
HRA |
Literature |
|||
| DEET (35%) stick/cream |
Low | A |
— |
— |
2.36E+01 |
— |
— |
— |
— |
— |
| Med. | S |
— |
— |
4.71E+01 |
— |
— |
— |
— |
— |
|
| High | C |
— |
— |
1.65E+02 |
— |
— |
— |
— |
— |
|
| DEET (75%) liquid |
Low | A |
— |
2.40E+03a |
5.36E+01 |
— |
— |
— |
— |
— |
| Med. | S |
— |
— |
1.07E+02 |
— |
— |
— |
— |
— |
|
| High | C |
— |
— |
3.75E+02 |
1.36E+02c |
— |
— |
— |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C =
chronic a Lowest-observed-effect-level (LOEL) based on LOEL from the literature b PDR D = potential dose rate for dermal contact; ADD = absorbed dermal dose c These data are based on occupational exposure of 15%–75% concentration of DEET over a period of 7 months. |
As indicated in the Table 139, no HRA route-specific dose estimates are available for acute oral exposure for comparison with dosage data reported in referenced literature.
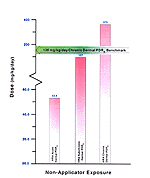
The literature cited for chronic dermal exposure is based on a concentration of DEET product ranging between 15–75 percent applied at >2 kg/7 months as compared with HRA dosage data that were based on concentrations of 75 percent and general application practices. [Figure 16]
E. Uncertainty/Variability of this Comparative Risk Characterization
Because of the limited documented human dose response or occupational exposure/health effects data, there is significant uncertainty with this risk characterization. However, nearly a half-century of human DEET usage with minimal health consequences adds authenticity to the results of the comparison of HRA data and literature studies.
F. Risk Communication Summary
DEET is generally believed to be of low toxicity. Although the dermal absorption of DEET has been known since its introduction, its use has continued because it is believed that it does not present a dermatologic or toxic hazard to humans.[787]
Benchmark dosage data are lacking for all exposure levels and routes. Based on the published benchmarks at which human health effects occur, the HRA calculated dose estimates at the high chronic dermal dosage levels are within a range that can cause rare health effects in humans.
Figure 16. Representation of Estimated Risk
| First Page | Prev Page | Next Page |