A. Acute Health Effects
1. Oral Exposure
In a reported suicide attempt, a 59-year-old male drank approximately 600 mL of 20 percent permethrin emulsion. Vomiting and diarrhea occurred after ingestion. On admission to the hospital, loss of consciousness and metabolic acidosis were observed. When he regained consciousness, the patient complained of a burning sensation in the oral cavity. He received fluid therapy after gastric lavage and recovered without severe complications. Apart from initially impaired consciousness, no clinical neurotoxicity occurred.[788] For acute oral exposure, this ingestion indicates an exposure level of 1623 mg/kg/d.
According to the Presidential Advisory Committee on Gulf War Veterans’ Illnesses, there are few reported cases of permethrin poisoning in humans, most likely because a large dose is required to cause poisoning. Humans rapidly detoxify and excrete permethrin. Clinical signs of immediate permethrin poisoning following large oral doses become evident within 2 hours and include incoordination, ataxia, hyperactivity, and convulsions, followed by prostration, paralysis, and/or death. The Committee found no reports of long-term effects from permethrin poisoning in humans.[789]
2. Dermal Exposure
A group of volunteers were given applications to an area of 4 cm� on the ear lobe of 0.05 mL of a field-strength preparation of technical (94–96 percent active ingredients) or formulated (32–36 percent) permethrin (0.13 mg/cm�) or of the inert ingredients. The intensity of paraesthesia induced by permethrin was fourfold stronger than that induced by a similar application of fenvalerate, permethrin being the least active compound for both the technical and formulated preparations. The inert ingredients elicited no cutaneous sensation. Paraesthesia appeared after a latent period of about 30 minutes, peaked between 8 and 12 hours, and disappeared after about 24 hours.[790]
One of 28 subjects with pediculosis pubis treated with a 1- percent permethrin rinse developed mild scrotal erythema and irritation 12 hours post-application. No dose estimate data were provided in the literature. Of 10 scabies patients treated with one application of 25 g (range, 21–32 g) of a 5 percent permethrin cream, followed by a thorough washing approximately 8–20 hours after treatment, six had limited, mild-to-moderate not pre-existing eczema on the scabies-affected skin at one or more examinations. For acute dermal exposure, this indicates an exposure level of 17.85 mg/kg/d. Permethrin has caused dermal irritation after topical exposure. [791]
Permethrin absorption from wearing treated (0.125 mg/cm�) military BDUs would be about 0.0006 mg/kg/d in humans. If the commercial 0.5 percent aerosol spray were used, human exposure would be markedly decreased, with the fabric treatment rate being reduced to about 0.025 mg/cm�. Though prelaundering treated fabrics removes up to 55 percent of the permethrin, it does not significantly alter the leaching rate during the first week of wear.[792]
The Extension Toxicology Network (EXTONET) indicates that permethrin is a moderately to slightly toxic pesticide in EPA Toxicity Class II or III, depending on the formulation. Formulations are placed in Class II because of their potential to cause eye and skin irritation.[793]
In a study by Farquhar, Hutchinson et al. (1981), permethrin exposure was performed on 10 individuals (4 men and 6 women). After applying 1- percent permethrin to the subjects, 30 percent developed skin irritation. In another study performed by Farquhar, Hutchinson et al., 10 male volunteers whose clothing had been impregnated with permethrin (3.8 mg/d) showed no signs of toxicity. A study by Le Quesne and Maxwell et al. (1980) that evaluated findings among 23 pest control workers occupationally exposed to multiple compounds, including permethrin. Although workers reported tingling, burning, and a rash starting at 30 minutes and lasting up to 8 hours postexposure, these findings were not exhibited among workers exposed to permethrin alone.[794]
After permethrin was introduced as an alternative to the treatment of head lice in humans, data were gathered on possible adverse effects from the use of a 1- percent permethrin creme rinse. Results reported from 18,950 individuals from 37 local public health departments showed few adverse reactions. The observed rate was approximately 2.2 adverse events per 1000 administrations.[795]
Pyrethroids, particularly permethrin and d-phenothrin, are safe and effective when used in recommended applications. Studies show that these compounds are potentially toxic at extremely high exposures; however, when used in conventional ways, only minor skin irritation in sensitive individuals results. Clinical manifestations subside after short periods when the inciting exposure is removed.[796]
3. Inhalation Exposure
Four of five workers in Sweden who packed conifer seedlings for 6 hours in a tunnel that had been sprayed 1 hour earlier with a 2 percent aqueous solution of permethrin, resulting in atmospheric concentrations of 0.011–0.085 mg/m� permethrin in the breathing zone, did not excrete detectable amounts of acid pyrethrin metabolites in the urine. One shorter individual whose face was close to the plants and who had the highest concentration of permethrin in the breathing zone excreted 0.26 �g/mL permethrin acid metabolites in the urine the following morning; in the afternoon, excretion was below the detection limit of the method. A group of five workers who planted the treated conifer seedlings were exposed to nondetectable- to low-permethrin levels in the breathing zone (mean, 2E-03 mg/m�; range, not detected — 6.0E-03 mg/m�) and excreted no detectable amount of permethrin metabolites in the urine.[797] For acute inhalation exposure, the lowest observed effect level based on the atmospheric concentration of 0.011 mg/m� was 9.4E-04 mg/kg/d. At the atmospheric concentration of 0.085 mg/m� the exposure level was 7.3E-03 mg/kg/d. No health effects were noted for either exposure level.
One known symptom from inhaled permethrin is cutaneous paresthesia described as burning, tingling, or stinging. This generally disappears within 1 day after removal of pyrethroids, but it persisted for more than 3 months in one case.[798] No dose estimate data were provided in the literature.
B. Subchronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
A study was performed of 22 pest control operators of whom 3 were specifically exposed to permethrin. The subjects were exposed to a normal commercial application of pyrethroid mix containing permethrin for 1–21 years. No blood, heart, lung, liver, or central nervous system abnormalities were documented. There was no correlation between the number of complaints and pyrethroid metabolite concentration in urine. Only fatigue was more common in the pyrethroid-exposed group. No specific pyrethroids were discussed.[799] No dose estimate data were provided in the literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
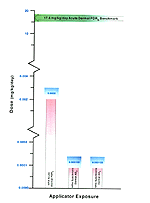
Table 140. Permethrin, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
| Pesticide | Exp. Level |
Type * |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa Literature |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| Permethrin 0.5% aerosol |
Low |
A |
— |
1.62E+03 c |
2.20E–03 |
1.78E+01d |
— |
— |
3.53E–06 |
7.28E–03 b |
Med. |
S |
— |
— |
— |
— |
1.03E–04 |
— |
3.53E–05 |
— |
|
High |
S |
— |
— |
— |
— |
1.03E–04 |
— |
3.53E–05 |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C =
chronic a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose b The study indicated that a 2% aqueous solution of permethrin had been sprayed, resulting in atmospheric concentrations of 0.011–0.085 mg/m� permethrin in the breathing zone. No human health effects were noted. c The data are derived from oral ingestion of a 20% permethrin emulsion. No HRA dose estimate data was available for comparison. d The study subjects received an exposure of 25 g of a 5% permethrin cream. |
Based on the Table 140, the HRA route-specific dose estimates are below the range where human health effects were reported in the literature. For example, a comparison of doses in an acute dermal exposure scenario shows that the dose at which no human health effects were observed is higher than the HRA estimated dose. When comparing acute inhalation doses, the dose at which no human health effects occur is above the HRA estimated dose. One area of exception is the acute oral exposure data that have no HRA dose estimate data available for comparison. [Figure 17]
Table 141. Permethrin, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
| Pesticide | Exp. Level |
Type * |
Route-Specific Dose (mg/kg/d) |
|||||||
| Oral | Dermal | Inhalation | ||||||||
| HRA | Literature | PDRDa HRA |
PDRDa Literature |
ADDa HRA |
ADDa Literature |
HRA | Literature | |||
Permethrin |
Low | S | — | — | — | — | 9.04E-04 | — | 1.94E-03 | — |
| Med. | S | — | — | — | — | 9.04E-04 | — | 2.67E-03 | — | |
| High | C | — | — | — | — | 1.19E-03 | — | 4.74E-03 | — | |
| * Exposure type: A = acute/subacute; S = subchronic; C =
chronic a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose |
No literature data are available for non-applicator doses to compare with the HRA route-specific absorbed dermal dose estimates.
E. Uncertainty/Variability of this Comparative Risk Characterization
Because of limited documented human dose response or occupational exposure/health effects data, there is significant uncertainty with this risk characterization. Minimal human data identified from the literature means extrapolated comparisons of health effects data were necessary. In addition, there is uncertainty because of the limited data on identified human test groups. For these reasons, caution should be exercised when drawing conclusions based on these data.
F. Risk Communication Summary
Benchmark dose study data are not available for all human health exposure levels and routes. The HRA calculated dose estimates reflected in the applicator and non-applicator tables are below doses that cause health effects in humans based on published benchmark data found in the scientific literature and reported in this study.
Figure 17. Representation of Estimated Risk
A. Acute Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
According to the World Health Organization (WHO), d-phenothrin has not been reported to cause human poisoning or other toxic effects in more than a decade of use. WHO concluded that d-phenothrin, when used as recommended, is not likely to pose a hazard to either human health or the environment. WHO also reported that d-phenothrin may be present in concentrations of up to 4 mg/kg in stored wheat, 0.8 mg/kg in flour, and 0.6 mg/kg in baked goods consumed by humans.[800]
2. Dermal Exposure
The material safety data sheets reviewed revealed mild human dermal hazard for d-phenothrin preparations.[801] Application of a 0.2 percent solution to head hair for 2 hours is a recommended standard treatment for head lice in the United Kingdom.[802] We speculate that a solution would not be recommended for treatment if it were known to cause health effects, especially in children, if it were inadvertently left in beyond the recommended time. Therefore, this treatment regimen represents an acute dermal no-observed-effect-level (NOEL) for humans. Assuming 1 ounce of shampoo would be applied to the head and skin for treatment of head lice, this would equate to approximately 56 mg on the skin (0.2 percent of 28 g). This would calculate to a potential dose rate for dermal contact of 0.8 mg/kg. Assuming a dermal absorption factor of 0.02 as stated in the Health Risk Assessment (HRA), this would calculate to about 0.016 mg/kg as an absorbed dermal NOEL for a 70-kg individual.
According to WHO, a 3-dose dermal application of 32-mg d-Phenothrin at 3-day intervals to human hair is a recommended treatment for lice. Using the calculations above this would amount to 96 mg on the human skin over 3 days, or a potential dermal dose of 1.3 mg/kg over the 3-day period (or 0.45 mg/kg/d [0.44–0.67 mg/kg/d reported]). No significant abnormalities, dermal irritation, clinical signs, or blood biochemical parameters were reported. The d-phenothrin was washed off 1 hour post-application and could not be detected in the blood at the minimum detection level of 6.0E-04 mg/kg.[803] An estimate of an acute absorbed dermal does of d-phenothrin would be 6.0E-03 mg/kg/d. The acute dermal absorbed range could then span a minimum of 6.0E-03 mg/kg/d to a possible maximum of 0.016 mg/kg/d for human body-lice treatment.
3. Inhalation Exposure
WHO reported that household aerosol spraying is not expected to create levels of exposure greater than 0.5 mg/m3 d-phenothrin. The initial concentration of d-phenothrin sprayed, how long this exposure persisted, and any indications of adverse health effects are unknown and were not reported. According to WHO, general human population exposure is expected to be very low, but precise data were lacking and values were not provided.[804]
Assuming a typical human inhalation exposure with no adverse effects is 0.5 mg/m3, an estimate of an acute human inhalation 24-hour NOEL is 0.17 mg/kg/d. A more conservative acute human inhalation NOEL is 7.1E-03 mg/kg assuming only 1 hour of exposure at 0.5 mg/m3 d-phenothrin.
The material safety data sheets reviewed indicated the acute inhalation hazards for d-phenothrin preparations were caused by other ingredients, propellants, and solvents rather than the active ingredient, d-phenothrin. The MSDSs made no reference of an occupational exposure limit (OEL) for d-phenothrin established by OSHA, NIOSH, ACGIH, or any other organization.[805] WHO indicated that with reasonable work practices, hygiene measures, and safety precautions, d-phenothrin is unlikely to pose an occupational health hazard.[806] No other acute inhalation human health effects studies were found in the research literature.
B. Subchronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not been found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Table 142. d-Phenothrin, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| d-Phenothrin 2% aerosol |
Low | A |
— |
— |
— |
— |
1.04E–04 |
6.0E-03b |
3.55E–05 |
7.10E-03b,c |
| Med. | S |
— |
— |
— |
— |
1.04E–04 |
— |
3.55E–05 |
— |
|
| High | S |
— |
— |
— |
— |
4.15E–04 |
— |
1.42E–04 |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C =
chronic a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose b No-observed-effect-level (NOEL) c Estimate of a conservative acute human inhalation. |
The acute dermal applicator exposure to d-phenothrin is estimated to be 1.04E-04 mg/kg/d in the HRA. A subchronic high d-phenothrin exposure is estimated at 4.15E-04 mg/kg/d in the HRA. Comparing this to the most conservative acute human dermal NOEL of 6.0E-03 mg/kg/d, we find the HRA dose estimate is 0.016 times a conservative standard dermal treatment for human lice eradication. Subchronic human dermal d-phenothrin data are unavailable, but the applicator exposure is estimated to be about 0.066 times the most conservative acute standard dermal treatment for lice eradication.
The acute inhalation applicator exposure to d-phenothrin is estimated at 3.55E-05 mg/kg/d — this is approximately 5.0E-03 times the estimated human inhalation NOEL of 7.1E-03 mg/kg/d. No subacute human NOEL was found in the literature, but the HRA estimation of 1.42E-04 mg/kg/d is 0.02 times the conservative estimated human inhalation NOEL of 7.1E-03 mg/kg/d. [Figure 18]
Table 143. d-Phenothrin, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa Literature |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| d-Phenothrin | Low | A |
— |
— |
— |
— |
— |
— |
6.50E–06 |
7.10E-03b,c |
| Med. | S |
— |
— |
— |
— |
— |
— |
9.61E–06 |
— |
|
| High | S |
— |
— |
— |
— |
— |
— |
1.89E–05 |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C =
chronic a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose b No-observed-effect-level (NOEL) c Estimate of a conservative acute human inhalation. |
Dermal post-application exposure scenarios were not calculated because of the low level of exposure estimated. This is scientifically sound for non-applicator dermal scenarios for d-phenothrin exposure. Based on human dermal treatment regimens and high margins of safety for applicator exposures in relation to them, non-applicator dermal exposure would be far lower and less significant to any dermal human health effects range.
E. Uncertainty/Variability of Comparative Risk Characterization
Because of a lack of documented dose response or occupational exposure/health effects data — except for dermal lice treatment — reliable characterization of human health effects with HRA estimations for inhalation of d-phenothrin is uncertain. The most confident human NOEL comparison is with the human dermal experience because d-phenothrin is routinely prescribed as a topical treatment for lice and the concentration range is known.
Human usage experience, however, indicates little hazard or acute human health effects through oral or inhalation routes. Unfortunately, there is a lack of human health effects data and no good documentation in the environmental and occupational literature reviewed to date. The amount of residue present in foodstuffs and the expected acute inhalation exposure for typical use of d-phenothrin provides an uncertain benchmark of some tolerable short-term inhalation exposure, but assumptions had to be made that this was a NOEL. This assumption itself interjects uncertainty.
Counteracting this, the lack of human health effects data after more than a decade of worldwide usage indicates a high degree of safety with normal use. Although not discussed here, animal data of a variety of species tends to support this assumption as well. Data have not been found indicating human subchronic or chronic effects by any exposure route.
F. Risk Communication Summary
The actual, documented human health effects database for d-phenothrin is extremely sparse. Data reviewed about human health effects, when compared with calculated exposures of Gulf War veterans, seem to be in the range for acute exposures, with a large and lower exposure difference for both dermal and inhalation exposure. Longer-term, subchronic exposures are also well below human acute NOELs, although subchronic human health effects values for adequate and direct comparison with this exposure are not available.
Based on what has been estimated and compared to date, evidence for acute and subchronic human health effects from exposure to the pesticide d-phenothrin has not been presented for any exposures of Gulf War veterans estimated in the HRA.
Figure 18. Representation of Estimated Risk
| First Page | Prev Page | Next Page |