A. Acute Health Effects
1. Oral Exposure
The US Agency for Toxic Substances and Disease Registry (ATSDR) reports a case of a 54-year-old female who died after ingesting an estimated 293 mg/kg dose of diazinon.[850] The woman died of petechial hemorrhages in the brain, stomach, and gastric tract. A research paper, citing a National Institute for Occupational Safety and Health (NIOSH) source from 1977, documented a lowest possible lethal human dosage of diazinon at 50 mg/kg, but the source of this value was not stated.[851]
Five individuals who ingested a range of 240–916 mg/kg diazinon suffered health effects but recovered.[852] Data indicate that death in humans can occur at 293 mg/kg and above, and possibly as low as 50 mg/kg. The 916-mg/kg level also represents an upper level for humans at which significant health effects occur, with recovery with prompt medical treatment.
An acute human dose response lowest-observed-effect-level (LOEL) for red blood cell (RBC) acetylcholinesterase (AChE) from exposure to diazinon has not been found in the literature. RBC AChE inhibition is considered a clinical marker of organophosphate exposure, including diazinon. Human plasma AChE inhibition will fluctuate more rapidly than RBC changes and will regenerate quickly after exposure to organophosphate pesticide active ingredients ceases.
Inhibition of 50 percent of human RBC AChE from normal levels is a level where symptoms of acute toxicity will appear.[853] Another source states that a 50 percent level of RBC AChE inhibition will present severe human cholinergic poisoning symptoms.[854] A scientific literature source has not been located documenting a 50 percent RBC inhibition to a human dosage of diazinon.
In a series of tests involving 2–4 human volunteers, a dose of 0.05 mg/kg/d diazinon was administered orally for up 28 days. In this study a 35–40 percent plasma cholinesterase (ChE) inhibition was presented; no effect of RBC cholinergic activity was found and no other adverse health effects were reported.[855] The range of 0.025–0.05 mg/kg/d can be considered a range where human plasma ChE suppression occurs, human RBC cholinergic activity does not, and no other health effects are reported.
In a study on human male volunteers, three individuals were dosed with 0.02 mg/kg/d and 0.025 mg/kg/d diazinon in cornstarch capsules for 38 and 43 days, respectively. Plasma and RBC ChE inhibition was assessed every 2–3 days. All three volunteers in the 0.025-mg/kg/d group showed consistent inhibition between 8 percent and 38 percent for plasma AChE relative to pretest values, and two of the three volunteers in the 0.02-mg/kg/d group showed consistent inhibition between 9 percent and 38 percent for plasma AChE. Volunteers did not present RBC AChE inhibition at either dosage, however. This study (as reported for a period of 1–7 days) concludes that the acute oral LOEL and no-observed-effect-level (NOEL) for plasma AChE inhibition in humans are 0.025 mg/kg/d and 0.02 mg/kg/d, respectively.[856]
2. Dermal Exposure
Dosage-related acute dermal studies of diazinon in humans have not been found in the research literature; however, there are reports of diazinon health effects from dermal contact. ATSDR reported that no studies were available that indicate that human deaths were related to dermal exposure to diazinon. Other scientific reports indicate that some humans have died from dermal contact with diazinon, so lethality by dermal contact, though unlikely, should be considered a potential health effect for humans. There are reports that diazinon caused allergic skin irritations from contact with human skin, but a dosage was not reported.[857]
ATSDR reported a study in which human volunteers were found to have a 3 percent–4 percent dermal absorption after 4–24 hours of a solution applied to the forearm and abdomen in acetone or lanolin grease.[858] If 4 percent diazinon is the accepted human absorption rate, the applied dermal dose to human skin is 25 times the absorbed dermal dose to produce similar toxic effects as those described by the oral route. In the absence of human dermal dose response studies, the suite of dosage-related human symptoms for acute, subchronic, and chronic oral toxicity for diazinon are assumed to be the same for dermal contact.
3. Inhalation Exposure
High inhalation exposure is expected to present AChE inhibition health effects in humans. In combination with dermal exposure, these health effects can include neurological symptoms leading to respiratory failure and cardiac arrest.[859] However, a dosage level in humans strictly from high inhalation exposure has not been found from literature sources reviewed so far.
Diethylthiophosphate (DETP), a diazinon metabolite, was found in the urinary samples of pesticide applicators.[860] A study of 99 applicators and supervisory non-applicator personnel measured exposure levels of 1.5 mg and 0.02 mg whole-body diazinon respectively per shift and showed an increase in DETP. Pre-shift measurements were carried out at the beginning of the study and at 39 days. Hence, the values present human effects information in an acute and initial subchronic range of exposure.
The study examined 18 physical frank health effects that included tiredness, dizziness, headache, muscle twitching, weakness in the hands and legs, tingling in the toes and fingers, depression, nausea, and tightness of chest. An entire battery of neurobehavioral tests was performed. Some postshift differences were observed in symbol digit speed and memory accuracy. The authors concluded that diazinon exposure was not responsible for the small performance shift observed in postshift symbol digit speed and memory accuracy because the performance deficit was not related to dose response. They further concluded that no evidence was found of adverse dose response related behavioral effects (including DETP level increase) among pest control workers who had low-level exposure to diazinon for relatively short durations.
Calculating an upper, lower, and mean dose range for applicators (which constitutes the higher exposed group) based on a 70-kg individual we arrive at 1.4E-03–0.15 mg/kg/d (0.1–10.4 mg/d exposure), with a median of 0.02 mg/kg/d (1.5 mg/d exposure). The median exposure is at the human oral NOEL, the upper bound measured exposure is approximately 7 times the NOEL with no indication of adverse health effects. The dose for post-application exposure ranged from 2.8E-05–2.8E-03 mg/kg/d (2.0E-03–0.2 mg/d exposure), with a median of 2.8E-04 mg/kg/d (0.02 mg/d exposure). Applicators wore personal protective equipment (PPE) ranging from gloves to respirators, so this exposure is not a measure of absorbed dose. The non-applicators did not wear PPE and these data are a measure of absorbed dose.
Given the scope of physical and neuropsychological tests, the number of subjects involved, and the fact that the study was based on diazinon alone and actual exposure was assessed, this study provides highly confident reassurance that the human NOEL established — and exposures below it — are absent physical and psychological effects for acute and subchronic time frames, including expression of a biomarker of exposure (DETP). Although this study had inhalation measurements, the whole-body diazinon exposure techniques that were used examined all dermal, oral and inhalation exposure routes. Therefore, it will be used for all three exposure routes.
Inhalation is assumed to be a combination of aerosol and vapor diazinon, particularly in application of the insecticide. High concentrations of diazinon capable of producing toxic effects purely from vapor inhalation under normal circumstances are not likely.
In a North Carolina study, air concentrations of diazinon in commercial pesticide buildings, service vehicles, and food-preparation areas following pesticide application were measured in ambient air. Storage and office rooms had a mean 284 ng/m3 air concentration of diazinon with a range of 85–837 ng/m3. Storage and office areas had the highest diazinon concentration. Another study found a maximum of 3.4 �g/m3 for a 14-hour period in a retail garden store that sold diazinon. Airborne levels of 38 �g/m3 diazinon were found in rooms 21 days after a crack-and-crevice application. Airborne diazinon levels peaked at 167 �g/m3 and 27 �g/m3 4 hours post-application. The airborne concentrations in this case indicated that building occupants should not enter treated rooms that are unventilated until 2 days post-application.[861]
A quick, calculated 24-hour dose would indicate a 2.9E-05 mg/kg/d dosage for the highest ambient sample (837 ng/m3), 0.013 mg/kg/d (38 �g/m3) in the 21-day crack-and-crevice treatment measurement, and a maximum dosage of 0.05 mg/kg/d if the maximum concentration of 163 �g/m3 4 hours after a broadcast spray application of diazinon remained constant over a day. The last example would be the only one where the human lowest observed adverse effect level (LOAEL) of 0.02 mg/kg/d would be exceeded.
The National Institute for Occupational Safety and Health (NIOSH) established a Recommended Exposure Level (REL) of 0.1 mg/m3 for diazinon for a 10-hour work shift, considered a safe working level, or NOAEL, of inhalation exposure for an average individual. Assuming inhalation of 1 m3 air/hour — and, thus, a dose of 1.0 mg/d divided by a 70-kg individual — we end up with an acute level (a single working day NOAEL) of 0.014 mg/kg/d for diazinon via the inhalation route in humans. This is well within a factor of 2 for the NOEL discussed for oral toxicity reported above and considered equivalent for toxicological comparison. The absorbed dose of diazinon by the inhalation route is considered to be 100 percent and the health effects ranges are considered equivalent to those listed for oral toxicity.
B. Subchronic Health Effects
1. Oral Exposure
A subchronic human dose-response LOEL for RBC AChE from exposure to diazinon has not been found in the literature. RBC AChE inhibition is considered a clinical marker of organophosphate exposure, including diazinon. Human plasma AChE inhibition will fluctuate more rapidly than RBC changes and will regenerate quickly after exposure to organophosphate pesticide active ingredients ceases.
Inhibition of 50 percent of human RBC AChE from normal levels is a point at which symptoms of acute toxicity will appear.[862] According to another source, a 50 percent RBC AChE inhibition level will result in severe human cholinergic poisoning symptoms.[863] No resource documenting a 50 percent RBC inhibition in humans has been found in the research literature.
In a series of tests involving 2–4 human volunteers, a dose of 0.05 mg/kg/d diazinon was administered orally for up 28 days. In this study, a 35–40 percent plasma ChE inhibition was presented, no effect of RBC cholinergic activity was found, but no other adverse health effects were reported.[864] A range of 0.020–0.05 mg/kg/d will be considered a subchronic oral dosage range at which human plasma ChE suppression occurs, human RBC cholinergic activity does not, and no other health effects are reported.
In a study on human male volunteers, three subjects received diazinon doses of 0.02 mg/kg/d and 0.025 mg/kg/d in cornstarch capsules for 38 and 43 days, respectively. This study, as applied to a three months-to-lifetime period, concluded that the subchronic oral LOEL for plasma AChE inhibition in humans is 0.02 mg/kg/d.[865] This LOEL is 0.005 mg lower than that in the acute study. No subchronic NOEL was stated for plasma AChE inhibition.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Subchronic studies describing human health effects strictly from diazinon inhalation were not found. The multiple-route diazinon metabolite study of pesticide applicators described in the acute inhalation exposure section above includes table values for subchronic toxicity, since the study was conducted over a 39-day period. The description of dose response conclusions is the same as in this previous description and are not repeated here.[866]
C. Chronic Health Effects
1. Oral Exposure
An adequate chronic dose response study of human health effects of diazinon from the oral exposure route, either toxicological or epidemiological in nature, is unavailable.[867]
A chronic oral LOEL is reported in the literature, which is supported by human data. In a study on human male volunteers, three individuals received diazinon doses of 0.02 mg/kg/d and 0.025mg/kg/d in cornstarch capsules for 38 and 43 days, respectively. This study, as applied to a three months-to-lifetime period, concluded that the chronic oral LOEL for plasma AChE inhibition in humans is 0.02 mg/kg/d. [868] This LOEL is 0.005 mg lower than in the acute study. No NOEL was stated for plasma AChE inhibition.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
According to the World Health Organization (WHO), no cases of chronic symptoms of delayed neurotoxicity in humans, even after diazinon poisoning by acute means, have been reported. The animal data, however, would indicate otherwise.[869]
3. Inhalation Exposure
Chronic dose response studies of human health effects of diazinon from the inhalation exposure route, either toxicological or epidemiological in nature, are unavailable.[870]
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Table 151. Diazinon, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
| Pesticide | Exp. Level | Type * | Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDaHRA |
PDRDa Literature |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| Diazinon 48% liquid (EC) (Handwand) | Low | A |
— |
— |
— |
— |
4.10E-06 |
2.0E-02 b |
7.15E-07 |
2.0E-02 b |
| Med | S |
— |
— |
— |
— |
5.12E-05 |
2.5E-02c |
8.94E-06 |
2.5E-02c |
|
| High | C |
— |
— |
— |
— |
1.91E-01 |
2.0E-02c |
1.43E-03 |
2.0E-02c |
|
| Diazinon 48% liquid (EC) (Backpack) | Low | A |
— |
— |
— |
— |
2.38E-05 |
2.0E-02 b |
7.15E-07 |
2.0E-02 b |
| Med | S |
— |
— |
— |
— |
2.98E-04 |
2.5E-02c |
8.94E-06 |
2.5E-02c |
|
| High | C |
— |
— |
— |
— |
1.98E-01 |
5.0E-02d |
1.43E-03 |
5.0E-02d |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose |
| b oral value; No-observed-effect-level (NOEL) |
| c oral value; lower bound subchronic human lowest-observed-effect-level (LOEL) |
| d oral value; upper bound subchronic human lowest-observed-effect-level |
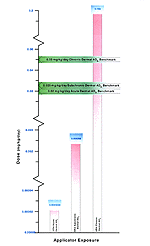
The high exposure estimated in the Health Risk Assessment (HRA) dermal applicator scenario assumed daily application of a large amount of pesticide (40 gallons) and no use of personal protective equipment. This scenario estimated an absorbed dermal exposure of 0.191 mg/kg/d and 0.198 mg/kg/d for handwand and backpack application. This exposure is about 10 times above the documented NOEL in humans for any physiological change. This value is 3 times the upper bound human LOEL of 0.05 mg/kg/d at which plasma AChE — but no RBC AChE inhibition and other frank health effects — has been documented in humans. It is not known at this time if this amount would cause significant (50 percent or more) RBC inhibition and lead to other cholinergic poisoning health effects.
If the medium or most likely diazinon applicator dermal exposure level for backpack power sprayers is examined, where more exposure is modeled to occur and where gloves and body dress are required, the highest modeled exposure is 2.9E-03 mg/kg/d. This is 0.015 times the NOEL of 0.02 mg/kg/d and 0.012 times the human LOEL of 0.025 mg/kg/d. Dermal exposures for hand sprayers are about 2.6E-03 times the 0.02 mg/kg/d human NOEL and 2.0E-03 times the 0.035 mg/kg/d human LOEL. The lowest modeled dermal diazinon application exposure scenarios are nearly 0.1 times the medium, putting the absorbed dermal diazinon dose at 1.6E-03 to far lower 1.0E-03 times the human NOEL and LOELs.
All inhalation diazinon exposures for applicators in the high (backpack sprayer) scenario are between 0.07 to 0.05 times the NOEL and LOEL respectively. The medium modeled inhalation exposures for applicators were 5.0E-04 times the NOEL, and the lowest modeled applicator exposures were more than 5.0E-05 times the NOEL for diazinon in humans. [Figure 22]
Table 152. Diazinon, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type* |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa Literature |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| Diazinon 48% liquid (EC) | Low | — |
— |
— |
— |
— |
— |
— |
— |
— |
| Med | — |
— |
— |
— |
— |
— |
— |
— |
— |
|
| High | C |
— |
— |
— |
— |
6.07E-02 |
2.0E-02 b |
8.31E-04 |
2.0E-02 b |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose |
| b oral value; Low-observed-effect-level (NOEL) |
The HRA estimated post-application high scenario (10 percent or less of field-deployed troops) modeled absorbed dermal doses of 0.0607 mg/kg/d diazinon, which is roughly 3 times the human NOEL of 0.02 mg/kg/d. This value is slightly above — and nearly equivalent with — the upper bound 0.05 mg/kg/d range at which human plasma AChE inhibition is observed from diazinon exposure but RBC and other health effects in humans are not.
E. Uncertainty/Variability of Comparative Risk Characterization
The LOEL/NOEL delta was only 0.005 mg/kg/d; therefore, one might expect greater sensitivity in some individuals, justifying a higher level of uncertainty in the NOEL, though not the LOEL. This uncertainty in the NOEL is further justified in that two of three subjects presented evidence of some plasma AChE inhibition at the lower dose, but not at the higher one.[871] This trend reverses the expected dose response curve.
However, greater certainty and less human variability are expected regarding the NOEL for human RBC AChE inhibition. At three low dosage levels, no human RBC AChE inhibition was presented in any test subjects at 0.02, 0.025, or 0.05 mg/kg/d diazinon-dosed groups. This is a fairly well-supported indictor in our review of the evidence (with three studies involving human volunteers, with three dosage levels) of the absence of RBC AChE inhibition as a health effect in humans at or below oral dosages of 0.05 mg/kg/d for acute and subchronic exposures and at or below a 0.02 mg/kg/d level for oral chronic exposure levels.
There are frank health effects due to cholinergic poisoning beyond AChE inhibition in humans. Qualitatively and quantitatively based on the human studies available, the acute oral levels of human health effects are the most certain. Oral human studies represent the data set with the largest number of dose response related studies.
Other benchmark levels of human health effects are less certain or do not exist. Because of this, oral studies were compared to the dermal and inhalation exposure routes. ATSDR concluded that scientifically supportive human data — primarily the oral route — exist for acute and subchronic (intermediate) exposures. Some of the human studies cited were uncertain because multiple routes of exposure or combinations of insecticides were involved. ATSDR noted in its review that there were no chronic toxicological and epidemiological studies in humans.[872] Therefore, the oral acute route provides the most certain characterized human health effects. Low-level diazinon exposure documenting the lack of health effects and plasma AChE inhibition is the best health effect characterization supported by human data. Other cholinergic toxic human symptoms are less supported by valid human dose response data and scientific literature.
For our characterization here, the absorbed dermal and inhalation doses are considered equivalent in toxic effects to the oral dosages described for humans. Based on the literature reviewed so far, below 0.05 mg/kg/d diazinon there is some confidence that the only human health effect is plasma AChE inhibition — there is no RBC AChE inhibition and no human health effects, behavioral or physiological, for acute and some subchronic time frames (e.g., 1 month).
F. Risk Communication Summary
1. Application Exposure Scenarios
For the modeled high application exposure scenarios, if diazinon exposures are in the range the HRA estimated, an exposure is possible that could cause suppression of the AChE plasma biomarker in humans. Other health effects are not comparable from human data at this time, and it is not certain that this exposure would significantly inhibit RBC AChE and, thus, give rise to other cholinergic health effects. It is also indicated in the HRA that this type of a high diazinon application scenario is rare because DoD prescribes PPE for this job.
All other diazinon application scenarios in the HRA, encompassing the medium and low ranges for the dermal and inhalation routes, are well below the human-based NOEL and LOEL. These scenarios are considered likely because DoD pesticide application protocols require PPE. Health effects would not be expected for troops that applied diazinon under these conditions.
2. Post-Application Exposure Scenarios
The largest numbers of troops were exposed passively to diazinon in the mess hall or latrine (the post-application scenario). If the HRA model is correct, the high post-application scenario would represent an area where human plasma AChE inhibition might be observed. This level is barely above the range at which there are human data to indicate absence of RBC AChE or other human health effects.
Medium and low absorbed dermal and inhalation post-application doses of diazinon are not modeled.
Figure 22. Representation of Estimated Risk
X. MALATHION
A. Acute Health Effects
1. Oral Exposure
In humans, the symptoms of an acute oral exposure to this cholinesterase-inhibiting compound include nausea, abdominal cramps, sweating, blurred vision, numbness, tingling sensations in the extremities, motor incoordination, headache, dizziness, tremor, difficulty breathing or respiratory depression, and slow heartbeat. Severe poisoning may result in unconsciousness, loss of bowel and bladder control, convulsions, and/or death. The health effects depend on the gender and amount of protein in the diet of an individual.[873] The lowest published lethal oral dose for a man is 471 mg/kg, while the lethal oral dose for a woman is about half that amount at 246 mg/kg.[874]
In one reported case of acute oral poisoning, a 24-year-old male was found deceased with two partially filled bottles of a malathion-containing mixture. A day or two before his death, the man suffered from severe diarrhea and vomiting. The official cause of death was ruled myocardial failure due to acute malathion poisoning. The blood, urine, bile, gastric, and intestinal contents of the deceased were tested for malathion content. Malathion was not detected in the blood or urine samples; however, it was present in the bile at a concentration of 570 mg/L, in the gastric contents at 201 g/kg, and in the intestinal contents at 98 g/kg. The contents of the bottles found near the deceased were tested for malathion. An exact concentration was not issued, but one bottle contained 11 percent malathion and the other contained 54 percent malathion.[875]
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
B. Subchronic Health Effects
1. Oral Exposure
According to the US Environmental Protection Agency’s Integrated Risk Information System (IRIS) Database, 5 healthy male volunteers, ranging in age from 23 to 63 years, participated in a study by Moeller and Rider (1962). The volunteers were given malathion in gelatin capsules at three dose levels: 8 mg/d for 32 days, 16 mg/d for 47 days, and 24 mg/d for 56 days. Cholinesterase (ChE) activity was measured in the subjects twice weekly before, during, and after administration of this pesticide. The intermediate dose was determined to be a no-observed-effect-level (NOEL) of 0.23 mg/kg/d. The high dose caused a depression in plasma and red blood cell (RBC) ChE activity, although no clinically manifested side effects were observed. This dose, the lowest-effect-level (LEL), was 0.34 mg/kg/d.[876]
In another study, a group of human volunteers were fed very low doses of malathion for a month-and-a-half. During this period and afterwards, there were no significant effects on RBC ChE activity for this group. The exact dosage given in this study was not reported.[877]
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Table 153. Malathion, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type* |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDe HRA |
PDRDe Literature |
ADDe HRA |
ADDe Literature |
HRA |
Literature |
|||
| Malathion 57% liquid (EC) Handwand | Low | A |
— |
— |
5.12E–04 |
— |
5.12E-05 |
3.40E–01ab |
3.57E–06 |
3.40E–01abc |
| Med. | S |
— |
3.40E–01a |
2.56E–03 |
— |
2.56E-04 |
3.40E–01ab |
1.79E–05 |
3.40E–01ab |
|
| High | C |
— |
— |
7.15E+00 |
— |
7.15E-01 |
3.40E–01ab |
2.14E–03 |
3.40E–01abd |
|
| Malathion 57% liquid (EC) Backpack | Low | A |
— |
— |
2.98E–03 |
— |
2.98E-04 |
3.40E–01ab |
3.57E–06 |
3.40E–01abc |
| Med. | S |
— |
3.40E–01a |
1.49E–02 |
— |
1.49E-03 |
3.40E–01ab |
1.79E–05 |
3.40E–01ab |
|
| High | C |
— |
— |
7.43E+00 |
— |
7.43E-01 |
3.40E–01ab |
2.14E–03 |
3.40E–01abd |
|
| Malathion 91% liquid (ULV) | Low | A |
— |
— |
5.22E–02 |
— |
5.22E-03 |
3.40E–01ab |
2.73E–04 |
3.40E–01abc |
| Med. | S |
— |
3.40E–01a |
1.59E–01 |
— |
1.59E-02 |
3.40E–01ab |
8.31E–04 |
3.40E–01ab |
|
| High | C |
— |
— |
4.64E+01 |
— |
4.64E+00 |
3.40E–01ab |
1.92E–02 |
3.40E–01abd |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a Lowest-observed-effect-level (LOEL) based on the lowest-effect-level (LEL) from the research literature |
| b Based on subchronic oral data; assumes equivalent absorption. |
| c No acute data are available. This data point is based on the LEL from a 56-day subchronic study. |
| d No chronic data are available. This data point is based on the LEL from a 56-day subchronic study. |
| e PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
Based on the Health Risk Assessment (HRA)/literature comparison table for application exposure shown above, the HRA calculated route-specific doses are well below the doses correlated with human health effects that appear in the research literature. The exception is at high exposure levels with dermal absorption. At this level the HRA estimates range from 2 to 10 times the benchmark. An example of the HRA dose being well below the research literature value occurs when comparing the HRA estimated dose (1.79E–05 mg/kg/d) for subchronic inhalation of vapor from 57 percent liquid malathion (handwand application) to the level at which health effects occurred in the study by Moeller and Rider (1962).[878] The HRA dose is nearly 5.3E-05 times the dose at which health effects were observed. In the high exposure with dermal absorption scenario, the literature value and the HRA dose are nearly equal. In these comparisons, the absorption characteristics were assumed to be the same in the oral, dermal, and inhalation exposure routes. [Figure 23]
The HRA estimated doses for post-application exposure scenarios (see table below) are also well below the levels at which health effects would be observed. The HRA subchronic dose at a medium exposure level is about 0.01 times the dose that caused health effects in the research literature, and at high exposure it is about 0.02 times the observed dose. Again, it should be noted that these comparisons were made using subchronic oral doses and not inhalation doses.
Table 154. Malathion, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type* |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDd HRA |
PDRDd Literature |
ADDd HRA |
ADDd Literature |
HRA |
Literature |
|||
| Malathion 57% liquid (EC) | Low | — |
— |
— |
— |
— |
— |
— |
— |
— |
| Med. | — |
— |
— |
— |
— |
— |
— |
— |
— |
|
| High | C |
— |
— |
3.03E+00 |
3.40E–01ab |
— |
— |
5.44E–04 |
3.40E–01ab |
|
| Malathion 91% liquid (ULV) | Low | A |
— |
— |
— |
— |
— |
— |
3.28E–03 |
3.40E–01abc |
| Med. | S |
— |
— |
— |
— |
— |
— |
3.28E–03 |
3.40E–01ab |
|
| High | S |
— |
— |
— |
— |
— |
— |
6.65E–03 |
3.40E–01ab |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a Lowest-observed-effect-level (LOEL) based on the lowest-effect-level (LEL) from the research literature |
| b Based on subchronic oral data; assumes equivalent absorption. |
| c No acute data are available. This data point is based on the LEL from a 56-day subchronic study. |
| d PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
E. Uncertainty/Variability of Comparative Risk Characterization
Because of a lack of reportable dose response data, there is significant uncertainty with this risk characterization. Data for dermal and inhalation scenarios were not available and an assumption was made that the literature values for acute and subchronic oral exposure would have the same values for inhalation exposure as long as the absorption rate was the same. In addition, there is uncertainty because of limited data on identified human test groups.
F. Risk Communication Summary
Based on the published benchmarks on human health effects used in this study, the HRA calculated dose estimates seem to be well below doses that cause health effects in humans. However, because of a lack of benchmark doses for all exposure levels and routes, caution should be exercised when determining comparing human health effects to HRA estimated dosages for this compound.
Figure 23. Representation of Estimated Risk
| First Page | Prev Page | Next Page |