VII. DICHLORVOS
A. Acute Health Effects
1. Oral Exposure
Health effects from ingesting dichlorvos may include pallor, nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, eye pain, blurred vision, constriction or dilation of the eye pupils, tearing, salivation, sweating, and confusion. These symptoms can occur from within a few minutes to 12 hours after the initial exposure. Severe poisoning affects the central nervous system by producing slurred speech, reflex loss, weakness, fatigue, involuntary muscle contractions, twitching, tremors of the tongue or eyelids, and eventually paralysis of arms, legs and respiratory muscles. Loss of bowel and bladder control, psychosis, irregular heartbeats, unconsciousness, convulsions, coma, and death through respiratory failure or cardiac arrest may also occur with severe poisoning.[818] No benchmark dosages are reported for the above symptoms.
2. Dermal Exposure
Acute exposure through skin contact may cause localized sweating and involuntary muscle contractions within 15 minutes to 2 hours after exposure.[819] Acute health effects from dermal contact with dichlorvos include the same health effects as those observed in oral exposure, with symptoms occurring a few minutes to several days after the initial contact.[820] No dermal benchmark dosages were reported for the above symptoms.
3. Inhalation Exposure
Acute health effects from inhalation of dichlorvos include the same as those observed in oral and dermal exposure. Inhaling dichlorvos may also produce a feeling of tightness in the chest, wheezing due to bronchospasm, runny nose, and frontal headache.[821]
According to a 1972 report, a research team subjected a group of human volunteers to a single exposure of dichlorvos 1.0 mg/m3 for 7.5–8.5 hours. The plasma cholinesterase levels in these individuals dropped 20–25 percent.[822] Assuming that the dose was administered to a 70-kg individual over an 8-hour period, the acute human LOEL would be 0.114 mg/kg/d.
4. Multiple Exposure Routes
A 1984 investigation reported by the US Environmental Protection Agency describes the effects of dichlorvos on applicators and residents following fumigation of houses. The applicators were exposed to average levels of 0.21 mg/m3 for approximately 25.5 minutes. Assuming that the dose was administered to a 70-kg individual over a 25.5-minute period, the acute human LOEL would be 1.2E-03 mg/kg/d. The residents of the fumigated homes were exposed to an average concentration of 0.21 mg/m3 for about 15.8 hours. Based on these data, the dose for a 70-kg individual would be 0.047 mg/kg/d. Data on two of the dichlorvos applicators showed decreases in plasma cholinesterase activity of 21 percent and 59 percent, respectively. The mean plasma cholinesterase activity for the residents a day after the application of dichlorvos was reduced only slightly (-7.9 percent) when compared with cholinesterase levels before the pesticide application. The only clinical effect noted in the residents was headache.[823]
B. Subchronic Health Effects
1. Oral Exposure
In a 1967 study cited by the USEPA, groups of five males were fed 1.0, 1.5, 2.0, or 2.5 mg doses of dichlorvos once a day for 28 days. Plasma cholinesterase activity was 71 percent of controls at the end of the 28 days in the group receiving 2.0 mg/d and was 70 percent of controls at the end of 20 days in the group administered 2.5 mg/d. There was no significant effect on erythrocyte cholinesterase activity, and there were no clinical symptoms of exposure in the subjects.[824]
In another EPA-cited study, 107 male volunteers were administered oral doses of dichlorvos ranging from 0.1 mg/kg to 16.0 mg/kg. Another group of 44 men received placebo pellets. Clinical symptoms reported by some of the treated and control groups were stomach rumbling, nausea, and diarrhea. Laboratory tests showed a decrease in cholinesterase activity in the treated subjects, and the decrease in this activity became greater as the dose increased. Erythrocyte cholinesterase activity also decreased with increasing dichlorvos administration, but the decrease was less than that observed in the plasma. The experiment had to be terminated in most subjects after a week due to the dramatic rate of decrease in plasma and erythrocyte cholinesterase activity from the daily dosings. An attempt to gradually increase the dose in the subjects produced similar cholinesterase depression; at 16 mg/kg, the experiment was terminated after only 5� days.[825]
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
The USEPA reports a limited occupational study in which six formulators and reactor workers in a pesticide manufacturing plant were exposed to dichlorvos for 20 days at an average air concentration of 0.1 ppm. The research team in this study observed a decrease in plasma cholinesterase activity and Vitamin A levels in 5 workers, noting that these levels were recovering slowly after a 13-day period of nonexposure.[826]
However, in another study, volunteers were exposed to dichlorvos concentrations ranging from 0.14 mg/m3 to 0.33 mg/m3 for 30 minutes every hour for 10 hours. This study was performed over 14 consecutive days. At the end of the 2-week period, the researchers found no change in cholinesterase activity in the volunteers.[827]
C. Chronic Health Effects
In most aspects, health effects may be the same for chronic dichlorvos as those for acute exposure for each exposure route. More specific chronic exposure effects may include impaired memory and concentration, disorientation, severe depression, irritability, confusion, headache, speech difficulties, delayed reaction times, nightmares, sleepwalking, and drowsiness or insomnia. Flu-like symptoms may also occur.[828]
Human health effects studies providing toxicology benchmark dosages for the chronic oral, dermal, and inhalation exposure routes were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Because there are no Health Risk Assessment (HRA) doses reported for a scenario in which applicators are exposed to dichlorvos, a comparison of HRA modeled doses to literature-cited benchmark health effect levels cannot be made. A LOEL value for an acute exposure to dichlorvos was calculated from the research performed by Gold et al. (1984).[829] The primary route of exposure was probably inhalation in this study, but dermal and oral exposure could also have occurred.
Table 148. Dichlorvos, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDb HRA |
PDRDb |
ADDb HRA |
ADDb Literature |
HRA |
Literature |
|||
| Dichlorvos 20% resin strip | Low | S |
— |
— |
— |
— |
— |
— |
6.01E-03 |
2.36E–02a |
| Med. | S |
— |
— |
— |
— |
— |
— |
3.32E-02 |
2.36E–02a |
|
| High | S |
— |
— |
— |
— |
— |
— |
9.62E-02 |
2.36E–02a |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a No-observed-effect-level (NOEL) data based on a 14-day inhalation exposure study. |
| b PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
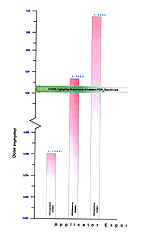
In the post-application exposure scenarios, the HRA estimated doses were very similar to the levels at which health effects occurred in the research literature. At low exposures, the HRA estimated dose is only 0.25 times the benchmark dose for inhalation exposure. At the medium level exposure, the HRA dose and the benchmark dose are nearly equal, while at the high level exposure the HRA estimated dose is slightly above the benchmark inhalation dose. It should be noted again that the HRA doses were compared with a NOEL from a subchronic inhalation study [Figure 20].
E. Uncertainty/Variability of this Comparative Risk Characterization
The accuracy of the comparison of HRA doses to benchmark doses in the post-application exposure study is uncertain. The subchronic NOEL (inhalation) dose is higher than the acute LOEL (multiple route) dose. These data were extrapolated from two separate studies that had small test groups.
F. Risk Communication Summary
Based on the published benchmarks at which human health effects occur used in this study, the HRA calculated dose estimates seem to be very close to the doses that cause health effects in humans. However, because this study encompasses only a small amount of benchmark doses for all exposure levels and routes from human health studies, caution should be exercised when comparing human health effects to HRA modeled exposure estimates for this compound.
Figure 20. Representation of Estimated Risk
VIII. CHLORPYRIFOS (3,5,6-TRICHLORO-2-PYRIDINYL
A. Acute Health Effects
1. Oral Exposure
According to the Agency for Toxic Substances and Disease Registry, "short-term oral exposure (one day) to low (milligrams) levels of chlorpyrifos causes dizziness, runny nose, confusion, salivation, and rapid heart rate. Short-term oral exposure to moderate (grams) levels of chlorpyrifos may cause paralysis, seizures, loss of consciousness, and death. Reports … also show that short-term exposure to chlorpyrifos may cause muscle weakness weeks after the original symptoms have disappeared. This is typical of organophosphate-induced delayed neuropathy (OPIDN). In humans, few cases of true OPIDN have been observed. However, true OPIDN has been seen in laboratory animals following very high doses of the pesticide. No permanent effects of short-term chlorpyrifos exposure in people have been found."[830]
Two human studies were conducted to determine specific dosages at which human health effects begin to occur. In the first study, by Coulston et al. (1972), male volunteers (4/dose group) were given daily oral doses of 0, 0.014, 0.03, or 0.1 mg chlorpyrifos/kg/d. The initial research protocol proposed that the doses be given for 7 weeks, 9 days, 21 days, and 28 days, respectively. It was reported on Day 9, however, that individuals of the 0.1-mg/kg group reached plasma cholinesterase (ChE) inhibition levels of 36 percent–82 percent and one individual complained of blurred vision, runny nose, and faintness. The report concluded that no significant plasma ChE inhibition was observed in the 0.03-mg/kg group after 21 days or in any of the other test groups. Plasma ChE levels returned to normal 25 days postexposure.[831]
Human no-observed-effect-levels (NOELs) and lowest-observed-effect-levels (LOELs) for repeated doses were derived from this study and published based on human data.[832] For plasma ChE inhibition, the NOEL is 0.03 mg/kg/d and the LOEL is 0.1 mg/kg/d. For red blood cell (RBC) acetylcholinesterase (AChE), the NOEL is 0.1 mg/kg/d, but no LOEL could be derived based on available data. There is uncertainty from this study because of the small test population (4 men/dose group) and the wide range of variability observed in the plasma cholinesterase inhibition (36 percent–82 percent in the 0.1-mg/kg/d group). In addition, the investigators attributed the health effects observed in the one individual to common cold, but the US Environmental Protection Agency’s Hazard Identification Assessment Review Committee (HIARC) concluded that a cold could not have caused the blurred vision.[833]
The second human study, conducted by Nolan et al. (1984), was performed using six Caucasian male volunteers ages 27–50 years. Each volunteer was given a single oral dose of 0.5 mg/kg in pellet form.[834] The volunteers were subsequently monitored for 30 days to determine plasma ChE and RBC AChE inhibition as well as the chlorpyrifos primary metabolite 3,5,6-trichloro-2-pyridinol (3,5,6-TCP) concentrations in the blood and urine. At no point during the 30 days were any clinical symptoms of toxicity or RBC AChE inhibition observed. Although the original paper reported no RBC AChE inhibition, a later report on the study by the EPA noted that peak AChE inhibition of 11 percent–52 percent was reached on Day 4.[835] This conclusion seems to be based on a graph presented in the original paper, but the EPA provides no reasoning for the conclusion. To remain consistent with published data, the conclusions made by Nolan et al. (1984) are used in this review. The maximum mean plasma cholinesterase inhibition observed was 15� 1 percent compared with baseline levels. Based on 3,5,6-TCP data, it was estimated that 72� 11 percent of the oral dose was absorbed.
This study, in conjunction with the study mentioned above, led to publication of human NOEL and LOEL data for single-dose exposures.[836] For plasma ChE inhibition, the NOEL is 0.1 mg/kg and the LOEL is 0.5 mg/kg. For RBC AChE, the NOEL is 0.5 mg/kg. This study is subject to the same uncertainties discussed previously, such as a small test population and variability of observed ChE inhibition among volunteers. This will become increasingly apparent when analyzing the dermal absorption study cited below, which was conducted using the same six volunteers.
Based on the observation of Hayes et al. (1980) that clinical manifestations of acute human toxicity occur when RBC AChE is inhibited at 50 percent of the baseline, an acute oral dose greater that 0.5 mg/kg would be required for adverse health effects.[837] Unfortunately, there is a lack of data to statistically support this observation. Most reports of chlorpyrifos-induced human toxicity lack dosage data, exposure route information, or discussion of potential contributing factors. Another question arises when referring to the neurological effects reportedly caused by chlorpyrifos and other organophosphate pesticide active ingredients. Investigators in the two human studies did not test for neuropsychological effects, and in cases where clinical tests were run the doses and exposure routes are not available.
OPIDN is associated with exposure to a few specific organophosphates, possibly including chlorpyrifos at very high doses. This syndrome is caused by the inhibition of neurotoxicity target esterase (NTE) in neural tissue, which triggers the degradation in sensory and motor axons of the peripheral nerves and/or spinal chord.[838] The inhibition of NTE occurs within hours of intoxication, and OPIDN has only been reported in cases where the dose was extremely high.[839]
In one reported case, a 42-year old male ingested ~300mg/kg chlorpyrifos in an attempted suicide.[840] Plasma cholinesterase activity measured 36 hours postexposure was nearly zero and the individual remained comatose with cholinergic symptoms for 17 days. Day 30 measurements of RBC and plasma cholinesterase as well as NTE were all significantly below normal levels, but began to rise from that point on. "On Day 43, the patient complained of weakness and paresthesias in the legs and symmetrical reduction of tendon reflexes was noted. Sensory conduction velocity was reduced and signs of denervation of some muscles [were] recorded. On Day 62, the leg weakness was more severe, the patient showed some gait impairment, and the tendon reflexes were absent. On electomyograph, the muscles of the legs showed symmetrical signs of denervation with signs of spontaneous activity." Further examination showed reduction of motor and sensory conduction velocities as well as signs of axon and myelin degeneration. Other cases of attempted suicide and accidental chlorpyrifos exposures with similar symptoms have been reported, but definitive doses are not provided.[841]
2. Dermal Exposure
Nolan et al. (1984) conducted a human dermal absorption study in connection with the oral study mentioned above, using the same six volunteers.[842] One received two 0.5 mg/kg dermal doses, one dissolved in methylene chloride and the other in dipropylene glycol methyl ether [DPGME], and the remaining five received doses of 5.0 mg/kg dissolved in DPGME. It was determined that 1.35� 1 percent of the dermal dose was absorbed based on blood chlorpyrifos measurements following exposure. The mean plasma ChE inhibition in the five volunteers was 13 percent, but further analysis showed a large discrepancy in levels between individuals. A 20 percent decrease was observed in one individual; there was no decrease in levels for two of the volunteers and a high variability in levels for the other two volunteers.
Based on the information from this study, if one were to assume that a maximum of 2.35 percent of the 5.0 mg/kg dermal dose was absorbed, that would give an absorbed dose of approximately 0.12 mg/kg. This 0.12 mg/kg dose could be the assumed absorbed human acute dermal LOEL even though two of the individuals showed no plasma ChE inhibition at this dose.
3. Inhalation Exposure
Fenske et al. (1990) performed measurements to determine the maximum air concentration of chlorpyrifos following residential broadcast applications.[843] Samples were taken from both ventilated and nonventilated rooms. Wipe samples taken within 0.5 hours following application were 1.69 �g/cm2 and 3.90 �g/cm2 in the ventilated and nonventilated rooms, respectively. Chlorpyrifos vapors measured 100 cm above the carpet never exceeded 20 �g/m3 in ventilated rooms, and maximum levels greater than 60 �g/m3 were measured in nonventilated rooms.
A poison control center report on chlorpyrifos exposures over a 10-year period provides four fatalities potentially caused by inhalation of high levels of chlorpyrifos.[844] Two separate exposures occurred in adult males while spraying chlorpyrifos under houses. Both experienced cardiopulmonary arrest that resulted in their deaths. No other causal factors are suggested and no dose estimates were provided. For these and other reasons, it is difficult to make conclusions with respect to these cases.
A third exposure occurred when, "an 85-year-old male was admitted to the hospital for treatment of pneumonia and sepsis." He had symptoms of lethargy, diarrhea, and fever. Four days before he was hospitalized, his home had been treated with a chlorpyrifos containing compound. One-week post application, soil samples taken from under the house contained 560 ppm chlorpyrifos and the air-conditioning filter contained 46 ppm. When the house was swabbed, chlorpyrifos levels were measured at 170 �g/ft2. His vital signs upon hospital admission were: heart rate 100 beats per minute; blood pressure 122/50 mmHg; temperature 99.6�F; and "rales were ausculated over the lungs." The patient died 13 days after being admitted to the hospital. Caution should be exercised when drawing conclusions from this case study because of the lack of a definitive dose, the age of the victim, and uncertainty of exposure route.
The fourth fatality occurred when a 39-year-old female inhaled an entire aerosol can containing chlorpyrifos, hydrocarbons, and pyrethrins. Her symptoms included cardiac arrest en route to the hospital, multiple seizures, neuromuscular abnormalities, and excessive pulmonary secretions. She died 16 days postexposure. Among the difficulties in drawing conclusions from this case study are lack of dose information, concentration of chlorpyrifos, and the presence of hydrocarbons and pyrethrins in the insecticide mixture.
Because of a lack of dose response data in humans for acute inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes, and use the acute oral LOEL of 0.1 mg/kg/d for Health Risk Assessment (HRA) comparison.
B. Subchronic Health Effects
1. Oral Exposure
Two human studies were conducted to determine specific dosages where human health effects begin to occur. In the first study, male volunteers (4/dose group) were given daily oral doses of 0, 0.014, 0.03, or 0.1 mg chlorpyrifos/kg/d.[845] The initial research protocol proposed that the doses be given for 7 weeks, 9 days, 21 days, and 28 days, respectively. However, on Day 9, individuals of the 0.1-mg/kg group reached plasma ChE inhibition levels of 36–82 percent and one individual complained of blurred vision, runny nose, and faintness. The report concluded that no significant plasma ChE inhibition was observed in the 0.03-mg/kg group after 21 days or in any of the other test groups. Plasma ChE levels returned to normal 25 days postexposure.
From this study, human NOELs and LOELs for repeated doses were derived and published.[846] For plasma ChE inhibition, the NOEL is 0.03 mg/kg/d and the LOEL is 0.1 mg/kg/d. For RBC AChE, the NOEL is 0.1 mg/kg/d, but no LOEL could be derived based on the available data. There is uncertainty in this study because of the small test population (4 men/dose group) and the wide range of variability observed in the plasma cholinesterase inhibition (36–82 percent in the 0.1-mg/kg/d group). In addition, the investigators attributed the health effects observed in the one individual to a common cold, but the HIARC concluded that a cold could not have caused the blurred vision.[847]
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the data from the acute dermal human studies will be used to make subchronic comparisons to the HRA exposure estimates.
3. Inhalation Exposure
Because of a lack of dose response data for subchronic inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes and use the subchronic oral NOEL of 0.03 mg/kg/d for HRA comparison.
C. Chronic Health Effects
1. Oral Exposure
For the purpose of this report, it will be assumed that the subchronic data collected from the human study reported above will remain constant and will be used in the HRA comparison.
2. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the data from the acute dermal human studies will be used for making chronic comparisons to the HRA exposure estimates.
3. Inhalation Exposure
Because of a lack of dose response data for chronic inhalation exposure, we will assume for the purpose of this review that equivalent absorption would occur in both oral and inhalation routes and use the subchronic oral NOEL of 0.03 mg/kg/d for HRA comparison.
D. Risk Characterization: Comparison of HRA-Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Table 149. Chlorpyrifos, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDf HRA |
PDRDf Literature |
ADDf HRA |
ADDf Literature |
HRA |
Literature |
|||
| Chlorpyrifos 45% liquid (EC) (handwand) | Low | A |
— |
1.00E–01a |
2.56E–04 |
5.00E+00a |
— |
1.20E–01a |
1.79E–06 |
1.00E–01ba |
| Med. | S |
— |
3.00E–02c |
— |
— |
3.84E–05 |
1.20E–01e |
8.94E–06 |
3.00E–02bc |
|
| High | C |
— |
3.00E–02cd |
— |
— |
7.15E–02 |
1.20E–01e |
7.15E–04 |
3.00E–02bcd |
|
| Chlorpyrifos 45% liquid (EC) (backpack) | Low | A |
— |
1.00E–01a |
1.49E–03 |
5.00E+00a |
— |
1.20E–01a |
1.79E–06 |
1.00E–01ba |
| Med. | S |
— |
3.00E–02c |
— |
— |
2.23E–04 |
1.20E–01e |
8.94E–06 |
3.00E–02bc |
|
| High | C |
— |
3.00E–02cd |
— |
— |
7.43E–02 |
1.20E–01e |
7.15E–04 |
3.00E–02bcd |
|
| Chlorpyrifos 19% liquid (ULV) | Low | A |
— |
1.00E–01a |
1.71E–03 |
5.00E+00a |
— |
1.20E–01a |
8.91E–06 |
1.00E–01ba |
| Med. | S |
— |
3.00E–02c |
— |
— |
1.02E–04 |
1.20E–01e |
1.77E–05 |
3.00E–02bc |
|
| High | S |
— |
3.00E–02cd |
— |
— |
2.61E–02 |
1.20E–01e |
3.60E–04 |
3.00E–02bcd |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic | |
| a Lowest-observed-effect-level (LOEL) | |
| b Based on acute oral data; assumes equivalent absorption. | |
| c No-observed-effect-level (NOEL) data based on a 21-day oral exposure study | |
| d No chronic data are available. This data point is based on the 21-day subchronic data. | |
| e No subchronic or chronic data are available. This is an acute LOEL from a single dermal dose. | |
| f PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
Based on the HRA/literature comparison table for application exposure, the HRA calculated route-specific doses seem to be well below the doses at which human health effects occur in the literature. For example, assuming equivalent absorption in both oral and inhalation routes, the HRA-estimated dose for acute inhalation of 45 percent liquid chlorpyrifos is approximately 1.8E-05 times the dose at which health effects would occur. In this same group, the HRA PDRD is 5.3E-05 times the dermal dose required to see human health effects. [Figure 21]
This same pattern holds true for the post-application exposure scenarios compared in the table below. At the highest estimated dose of 2.28E–02 listed under high chronic ADD, the literature-extrapolated dose at which human health effects occur is 4 times greater than the estimated dose. This is true only if we assume that subchronic and chronic exposures would result in the same human health effects observed following acute exposure. Based on these assumptions, all estimated doses seem to be well below the level at which human health effects have been reported in the literature.
Table 150. Chlorpyrifos, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDf HRA |
PDRD Literature |
ADDf HRA |
ADD Literature |
HRA |
Literature |
|||
| Chlorpyrifos 45% liquid (EC) | Low | — |
— |
1.00E–01a |
— |
— |
— |
— |
— |
1.00E–01b |
| Med. | — |
— |
3.00E–02c |
— |
— |
— |
— |
— |
3.00E–02bc |
|
| High | C |
— |
3.00E–02cd |
— |
— |
2.28E–02 |
1.20E–01e |
6.97E–05 |
3.00E–02bcd |
|
| Chlorpyrifos 19% liquid (ULV) | Low | A |
— |
1.00E–01a |
— |
5.00E+00 |
— |
1.20E–01 |
1.03E-04 |
1.00E–01b |
| Med. | S |
— |
3.00E–02c |
— |
— |
— |
— |
1.03E-04 |
3.00E–02bc |
|
| High | S |
— |
3.00E–02cd |
— |
— |
— |
— |
2.09E–04 |
3.00E–02bcd |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic | |
| a Lowest-observed-effect-level (LOEL) | |
| b Based on acute oral data; assumes equivalent absorption. | |
| c No-observed-effect-level (NOEL) data based on a 21-day oral exposure study | |
| d No chronic data are available. This data point is based on the 21-day subchronic data. | |
| e No subchronic or chronic data are available. This is an acute LOEL from a single dermal dose. | |
| f PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
E. Uncertainty/Variability of this Comparative Risk Characterization
Several human studies have been found in the literature involving more than one individual over multiple dose ranges to document human health effects from chlorpyrifos exposure. This reduces uncertainty of the dose response relationship for human health effects data regarding this compound. However, variability of human response was also documented in the literature cited. The certainty and confidence regarding chlorpyrifos human health effects benchmarks in this review is increased because they have been used by USEPA and FDA as the basis to establish more conservative chronic oral reference doses.[848,849]
F. Risk Communication Summary
Based on published benchmarks, the HRA calculated dose estimates seem to be well below doses where human health effects occur. It should be noted, however, that this is based on the assumptions communicated throughout this text and a statistically significant comparison is not possible because of a lack of sufficient dose response data.
Figure 21. Representation of Estimated Risk
| First Page | Prev Page | Next Page |