K. PROPOXUR
1. Acute Health Effects
a. Oral Exposure
Although dosages are not reported, human fatalities due to propoxur poisoning are rare.[930] Epidemiological information summarized by the U.S. Environmental Protection Agency’s Office of Pesticide Programs (OPP) Incident Data System (1992 through April 1996) contained descriptions of 91 human exposures to propoxur. Seventy of the 91 exposures were from two incidents, both of which involved post-application exposures. Symptoms from these post-application exposures included headaches, nausea, depression, and respiratory irritation. The California Pesticide Illness Surveillance Program (1982-1993) indicated that 125 persons exposed to propoxur showed systemic symptoms, with 63 presenting respiratory symptoms such as coughing, tightness in the chest, shortness of breath, and congestion. No human dosages for these reports were indicated in the EPA Registration Eligibility Decision (RED) document cited.[931]
The cumulative human health effects of propoxur can be additive to other carbamates because it is structurally similar to them. These carbamates include carbaryl, methomyl, carbofuran, methiocarb, aminocarb, and bendiocarb. Furthermore, other pesticide active ingredients may have common toxicity endpoints with propoxur.[932] Propoxur can be detected in blood immediately after exposure but is metabolized quickly in humans, with a half-life of 1 hour. It is mainly metabolized to 2-iso-propoxyphenol and is totally eliminated in urine within 1-3 days.[933]
An acute dietary exposure (1-day) endpoint of 0.15 mg/kg was selected from a published study involving human volunteers.[934] A 42-year-old 90-kg male who ingested 1.5 mg/kg presented symptoms of blurred vision, nausea, sweating, increased blood pressure, and vomiting. The effects were most pronounced 30-45 minutes after ingestion. This subject exhibited red blood cell (RBC) inhibition of 73 percent 15 minutes after ingestion. Two hours after ingestion, RBC cholinesterase (ChE) activity was essentially normal. A single dose of 0.36 mg/kg caused a 43 percent drop in RBC ChE activity, with transient stomach discomfort, blurred vision, moderate facial redness, and sweating. RBC ChE was normal within 3 hours. Five doses of 0.15 mg/kg or 0.2 mg/kg at half-hour intervals resulted in transient RBC ChE depressions. The 0.15-mg/kg dose resulted in the occurrence of 40 percent RBC ChE inhibition. No other health effects were discussed.
Based on human data the EPA Reference Dose (RfD)/Peer Review Committee (September 30, 1994) recommended that the LOEL of 0.15 mg/kg as a starting point for establishing a RfD. This level represented the lowest dose of propoxur tested under controlled conditions on humans. EPA believed a LOEL was appropriate because multiple doses of 0.15 and/or 0.2 mg/kg were associated with transient RBC ChE inhibition.[935]
In 1989, the FAO/WHO joint committee on pesticide residue (JMPR) conducted a review of propoxur and established an acceptable daily intake (ADI) of 0.02 mg/kg/d based on the acute no-effect level in humans. In the JMPR evaluation of the human study, the no-observed-effect-level (NOEL) was considered to be 0.2 mg/kg/d since the depression of erythrocyte cholinesterase did not exceed 20 percent and the recovery was very rapid. It seems, therefore, that the ADI value generated by the JMPR was based on the same human study used by the EPA in generating its RfD value. However, the EPA has criteria interpreting ChE inhibition as a health effect, which is different from those used internationally. The JMPR probably considered the LOEL as determined by the EPA to be a NOEL.[936]
Because the 0.02-mg/kg/d level is 0.1 times the 0.2-mg/kg/d level where mild RBC acetylcholinesterase (AChE) inhibition occurs, we concur with FAO/WHO that this is a NOEL extrapolated from human experience data. This is due partly to the fact that RBC inhibition can present health effects and occurs after plasma AChE inhibition. An experimentally verified propoxur oral NOEL, as interpreted as an upper-boundary oral exposure level where no plasma AChE inhibition is presented based on human data, has not been found in the research literature.
b. Dermal Exposure
Technical propoxur is considered to be Toxicity Category III or IV for dermal, inhalation, eye, and skin irritation. It is not considered a skin sensitizer.[937] An acute dermal human absorption study of propoxur is cited where six individuals received a single intravenous dose of radiocarbon-labeled propoxur. Total urine was collected for 5 days postdose and the percent of radiolabeled-dose excreted in the urine was determined. Subsequently, the same six individuals received a single dermal dose of radiocarbon propoxur for an exposure period of 24 hours. Total urine was collected postdose and the percent of radio labeled-dose excreted in the urine was determined. Corrected total excretion was 19.6 percent of the dose dermally administered.[938] Prior to this study, the EPA used a value of 50 percent dermal absorption from a rat dermal absorption study of propoxur. Based on human data in this study, the EPA in 1997 adopted 19.6 percent as the human dermal absorption factor.[939] Based on human data, the absorbed dermal dose would be approximately one-fifth the applied dermal dose.
The following comparison explains the differences and avoids confusion between a calculated dermal LOEL of propoxur and one derived from human experience and other data. If we multiply the oral toxicology endpoints LOEL (0.15 mg/kg/d) by 5 and by 70 kg, we estimate the acute dermal LOEL at about 50 mg/d. The FAO/WHO oral NOEL (0.02 mg/kg) produces a dermal NOEL value of about 7 mg. This calculation vastly overestimates risk and is not a correct extrapolation. The EPA recommends an occupational and residential dermal NOEL for humans at >1000 mg/kg/d, based primarily on a subchronic rabbit dermal study but also human use experience.[940] This is approximately a dermal exposure of 70 g, or roughly 2.5 oz per day, on skin with no human health effects expected.
The high dermal NOEL for propoxur is valid because propoxur is metabolized rapidly, detoxifying and reducing its AChE inhibition potential. The EPA concluded that risk assessments for chronic (non-cancer) and short-term dermal and inhalation exposures to propoxur are not required because no adverse effects were observed at the highest dose tested of 1000 mg/kg/d in a dermal study (rabbit), and because the vapor pressure of propoxur is extremely low and the registered uses of propoxur are such that significant human exposure via the dermal or inhalation route is not expected.[941] For the reasons provided above, an acute dermal NOEL for propoxur of 1000 mg/kg/d is used for comparison with the Health Risk Assessment (HRA).
c. Acute, Subchronic, and Chronic Inhalation
Because of judgments made in the research literature about the human health effects of propoxur, all three exposure time frames for inhalation are discussed here. For short-term (1-7 days), intermediate-term (1 week to several months), and chronic-term (several months to lifetime) inhalation occupational and residential exposures to propoxur, the EPA established a risk assessment toxicological endpoint for inhalation exposure at a NOEL of 2.2 mg/m3. This inhalation NOEL is based on significant plasma, RBC, and brain ChE inhibition in rats (MRIDs 342648001 and 43398501).[942]
An occupational study supporting the 2.2 mg/m3 NOEL involved 24 workers exposed to 2.0 mg/m3 propoxur (cited as the maximum workplace limit) for 4 hours. The study reported a 24 percent erythrocyte and 53 percent plasma AChE inhibition 4 hours after exposure ceased. Erythrocyte and plasma AChE returned to normal and no inhibition was present at the end of shift (8 hours). All propoxur was eliminated within 24 hours as 2-iso-propoxyphenol. The maximum amount of propoxur in the plasma postexposure correlated to a range with no reported symptoms.[943]
The EPA concluded that a risk assessment for inhalation exposure is not required because the vapor pressure of propoxur is extremely low and the registered uses of propoxur are such that significant human exposure via the inhalation route is not expected. Converting the 4-hr NOEL value, cited in the above paragraph, to mg/kg/d, we arrive at (2.0 mg/m3 � 1 m3/hr � 24 hr/d)/70 kg = 0.68 mg/kg/d. The EPA recommends using the 2.2-mg/m3 value for acute, subchronic, and chronic human exposure assessments to propoxur.[944] This calculates to 0.75 mg/kg/d. The EPA recommends this value because it is very close to the human study value and the EPA recommends using it for all exposure scenarios. The 2.0-mg/m3 value is conservatively used for comparison with HRA exposure assessments as a propoxur LOEL, using EPA criteria because some literature indicates AChE inhibition in humans in plasma above 20 percent occurs and some temporary erythrocyte depression is evident.
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Chronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 157. Propoxur, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
HRA data indicate that for applicators 0.00298 mg/kg/d is the low-exposure scenario acute potential dermal dose and 8.36 mg/kg/d is the high-scenario subchronic potential dermal dose. The low acute dermal dose estimated in the HRA is only 3.0E-05 times the 1000 mg/kg/d acute propoxur dermal NOEL.
HRA data indicate that for applicators a low-exposure scenario acute inhalation exposure is 0.00000357 mg/kg/d for inhalation and 0.00241 mg/kg/d for a high-scenario subchronic exposure. Using 0.68 mg/kg/d as a LOEL reference point based on human data, estimated exposures in the HRA are only 3.5E-03 times this value for the high scenario and less than 1.0E-05 time this LOEL for the low scenario (Figure 25).
Table 158. Propoxur, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The HRA data indicate a non-applicator medium dermal acute exposure of 1.52 mg/kg/d and a subchronic dermal exposure of 7.05 mg/kg/d. The high-inhalation post-application exposure scenario of 0.000132 mg/kg/d in the HRA compared with a 0.68 mg/kg/d LOEL.
5. Uncertainty/Variability of Comparative Risk Characterization
The EPA concluded that an additional uncertainty factor (UF) is not warranted for the propoxur chronic risk assessment or for estimating risk from acute or short-term exposures.[945]
The EPA used the 0.15-mg/kg endpoint for the human oral study as a starting point for a dietary RfD. An uncertainty factor of 10 was then applied to account for intraspecies variability, and an additional UF of 3 was applied to compensate for the lack of a NOEL. On this basis, the RfD was calculated to be 0.005 mg/kg/d. The EPA indicated that the acceptable daily intake (ADI) of 0.02 mg/kg/d established in 1989 by the FAO/WHO joint committee on pesticide residue (JMPR) was based on the acute no-effect level in humans.[946] The health effects endpoint of AChE in humans, at least through the inhalation route, was corroborated through a larger study of workers in addition to a controlled oral study on a smaller group of subjects. Fairly good dose response in humans is exhibited.
Because of correlation with real world data, reasonable confidence exists for post-application inhalation exposures calculated in the HRA. To calculate concentrations of propoxur in the air of treated houses, the EPA pooled air concentration data for all rooms to yield an average air concentration of 5.1 µg/m3. Absorption by the three inhalation routes was assumed to be 100 percent. The hours/day of inhalation exposure was the same as for dermal exposure. Total dermal and inhalation exposure was calculated at 1.4E-04 mg/kg/d. This value is in the same range as the post-application inhalation exposure calculated in the HRA 1.32E-04 mg/kg/d).
These two areas-lower uncertainty about exposure and human health effects-provide at least qualitatively higher degree of certainty.
6. Risk Communication Summary
In most cases, the comparison
between the exposures in the HRA to expected human health effects is several
thousand to 100,000 or more. For all scenarios, there is a comparative difference
of at least 100. With the health effects documented in our literature review,
no human health effects ranges are approached through either the dermal or inhalation
routes.
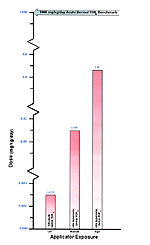
Figure 25: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Propoxur at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for acute dermal exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
L. BENDIOCARB
1. Acute Health Effects
Bendiocarb is absorbed through all the normal routes of exposure, but dermal absorption is especially rapid. Carbamates generally are excreted rapidly and do not accumulate in mammalian tissue. If exposure does not continue, cholinesterase (ChE) inhibition and its symptoms reverse rapidly. In nonfatal cases, the illness generally lasts less than 24 hours. Within two days after feeding doses of up to 10 mg/kg of bendiocarb to rats, 89-90 percent of the dose was eliminated in the urine, 2-6 percent was exhaled, and another 2-6 percent was eliminated in the feces. This same elimination pattern was observed in a human subject given an oral dose of bendiocarb.
Bendiocarb's toxicity is the result of its rapidly reversible cholinesterase inhibition. There is a wide dose margin between the onset of mild symptoms and lethal effects.[947] Symptoms of bendiocarb poisoning include weakness, blurred vision, headache, nausea, abdominal cramps, chest discomfort, constriction of pupils, sweating, muscle tremors, and decreased pulse. If there is severe poisoning, symptoms of twitching, giddiness, confusion, muscle incoordination, slurred speech, low blood pressure, heart irregularities, and loss of reflexes may also be experienced. Death results from discontinued breathing, paralysis of muscles of the respiratory system, intense constriction of the openings of the lung, or all three.
No occupational exposure limit for bendiocarb has been established by OSHA, ACGIH, NIOSH, or other organization.
a. Oral Exposure
In a series of experiments involving administration of one to three oral doses of 76 percent wettable powder formulation of bendiocarb to human subjects, levels ranged between 0.003 mg/kg and 0.200 mg/kg. A threshold dose for mild cholinergic symptoms was observed between 0.15 mg/kg and 0.20 mg/kg. A dose of 0.20 mg/kg was followed by mild vertigo, nausea, and sweating. In addition, this dose suppressed up to 40 percent red blood cell (RBC) acetylcholinesterase (AChE) activity. The maximum no-effect level was identified at 0.10 mg/kg. This lowest dose produced no detectable cumulative effects. Recovery from these effects at all dosages was notable after 30 minutes and complete within 4 hours.[948]
A case of self-induced fatal acute poisoning from oral ingestion of bendiocarb was reported in the research literature. A 27-year-old Caucasian male of approximately 70 kg body weight was found deceased at his home. Three empty sachets of bendiocarb were found together with a pint-size beer glass, and a tablespoon with evidence of residual wetted white powder was recovered. The sachets had each contained 15 g of wettable powder of 80 percent w/w bendiocarb. This equates to an approximate dose of 514.28 mg/kg. Determination of RBC ChE was not possible. In a case of fatal overdose involving a large quantity of carbamate insecticide, it is suggested that spontaneous hydrolysis would be so overwhelmed that significant ChE carbamylation would persist. This lethal dose response is unlike a more rapid reversal in sublethal carbamate poisoning.[949]
b. Dermal Exposure
Bendiocarb possesses a low dermal toxicity. It is not a skin irritant, and contamination by contact with spray deposits is negligible.[950] Human volunteers show the same pattern of rapid AChE inhibition and rapid recovery as exhibited in animal extrapolation studies. During the course of field studies it was shown that RBC ChE inhibition by bendiocarb is rapidly reversible and, therefore, routine ChE monitoring of operators is not necessary. Dermal absorption of bendiocarb is facilitated under hot and humid conditions, and special care must be taken when applying insecticides at these times. Effective training, adequate protective clothing, and good personal hygiene are requisite in achieving safe application of insecticides in house-spraying programs.[951]
Cumulative results from six safety studies demonstrated that skin contact with 3 mg/kg bendiocarb resulted in an asymptomatic but significant decrease in ChE activity of more than 25 percent from the pre-exposure levels. Skin contact with 0.1 mg/kg failed to produce significant changes in ChE activity.[952]
Bendiocarb is absorbed through all the normal routes of exposure, but dermal absorption is especially rapid. Carbamates generally are excreted rapidly and do not accumulate in mammalian tissue.[953]
c. Inhalation Exposure
Bendiocarb does not present a vapor hazard and is suitable for use in areas where a nonvolatile insecticide is important.[954]
In one case of exposure while applying bendiocarb, the victim experienced symptoms of severe headache, vomiting, and excessive salivation, and his ChE level was depressed by 37 percent. He recovered from these symptoms in less than 3 hours with no medical treatment and his ChE level returned to normal within 24 hours.[955]
In another case, poisoning occurred when an applicator that was not wearing personal protective equipment attempted to clean contaminated equipment. The victim experienced nausea, vomiting, incoordination, pain in his arms, hands and legs, muscle spasms, and breathing difficulty. These symptoms abated within 2 hours after decontamination and treatment with atropine. The victim fully recovered by the following day.[956]
2. Subchronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute oral human studies will be used for making chronic and subchronic comparisons to the Health Risk Assessment (HRA) exposure estimates.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute dermal human studies will be used for making chronic and subchronic comparisons to the HRA exposure estimates.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute oral human studies will be used for making chronic and subchronic comparisons to the HRA exposure estimates.
3. Chronic Health Effects
a. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute oral human studies will be used for making chronic and subchronic comparisons to the HRA exposure estimates.
b. Dermal Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute dermal human studies will be used for making chronic and subchronic comparisons to the HRA exposure estimates.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, data from the acute oral human studies will be used for making chronic and subchronic comparisons to the HRA exposure estimates.
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 159. Bendiocarb, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The HRA/Literature comparison table for application exposure identifies HRA calculated route-specific doses below those identified in the literature. If equivalent absorption is assumed for both oral and inhalation routes, the HRA estimated dose for acute inhalation of bendiocarb is approximately 2.9E-03 times the level where health effects were observed in the literature.
Careful consideration should
be given to comparisons of subchronic and chronic dermal doses. HRA calculated
doses are greater than those identified in the literature. Because of a lack
of documented dermal dose response and occupational exposure/health effects
data, acute data from a single dermal dose were extrapolated for comparison.
Therefore, reliable characterization of human health effects with HRA estimations
for subchronic and chronic dermal doses are uncertain (Figure 26).
Table 160. Bendiocarb, comparison of HRA doses to benchmarks (post-application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Data from the HRA enable only two comparisons with the dermal subchronic and chronic routes. According to the HRA-estimated subchronic and chronic dermal doses shown in the table 160, 3.79E-01 is greater than the literature-extrapolated doses at which human health effects occur. This assumption is true only if subchronic and chronic dermal exposures result in the same human health effects observed following acute exposure.
As reported in the HRA/Literature comparison table for application exposure, careful consideration should also be given to subchronic and chronic dermal doses. HRA calculated doses are greater than those identified in the literature. Because of a lack of documented dermal dose response and occupational exposure/health effects data, acute doses were extrapolated for comparison. Therefore, reliable characterization of human health effects with HRA estimations for subchronic and chronic dermal doses are uncertain.
5. Uncertainty/Variability of this Comparative Risk Characterization
Based on the absence of reportable human dose data, there is significant uncertainty with this risk characterization. Minimal human data identified from the literature necessitated extrapolated comparisons of subchronic and chronic health effects from acute doses and assumptions of equivalent route-specific absorption. In addition, there is uncertainty because of limited data on identified human test groups.
For these reasons, caution
should be exercised when drawing conclusions from these data.
6. Risk Communication Summary
As reported in the above HRA/Literature comparison tables for applicators and post-applicators, careful consideration should be given to subchronic and chronic dermal doses. HRA calculated doses are greater than those identified in the literature. Due to a lack of documented dermal dose response and occupational exposure/health effects data, acute data from a single dermal dose were extrapolated for comparison. Therefore, reliable characterization of human health effects with HRA predictions for subchronic and chronic dermal doses are uncertain.
Additionally, based on
the limited published benchmarks, the HRA calculated acute dermal dose and all
inhalation estimates seem to fall below doses at which identified human health
effects occur. It should be noted, however, that this is based on the assumptions
communicated throughout this text and a statistically significant comparison
is not possible because of a lack of sufficient dose response data.
Figure 26: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Bendiocarb at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative LOEL for acute dermal exposure route from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.
M. LINDANE
1. Acute Health Effects
a. Oral Exposure
Common effects of acute lindane poisoning are conjunctivitis, nausea, restlessness, headache, dizziness, and vomiting with muscle tremors, abdominal pain, and diarrhea. Severe poisoning may result in seizures, convulsions, coma, and death.[957] Symptoms of acute exposure in humans usually occur within 1 hour of the dose.[958]
Research professionals apparently disagree on the lethal dose of lindane. Some have stated that 10-20 mg/kg can be lethal to humans but higher concentrations can be tolerated.[959] Others have estimated the lethal dose to be about three grams.[960] This disparity most likely is due to variations of physical and physiological characteristics in humans.
Ohly (1973) reported a suicide attempt by a person who ingested the equivalent dose of 309 mg/kg. The individual suffered severe convulsions within the first 24 hours of ingesting the pesticide. The poisoning caused severe toxic damage to the liver, but the patient fully recovered after 5 weeks of treatment.[961]
A National Institutes of Health (NIH) Toxnet publication referenced a study in which humans tolerated a dose of 40 mg lindane per day for 14 days with "no ill effects."[962] Because of an inability to obtain the cited peer-reviewed reference, it is difficult to determine what "no ill effects" means. This statement could refer to cholinesterase inhibition levels or clinically observed symptoms. In order to be conservative, it will be assumed that the reference refers to observable frank human health effects. There is also uncertainty because of a lack of information on dose administration data and size of the test group. With these limitations in mind and a lack of other published human dose response data, the 40-mg/d dose will be used in the comparative Health Risk Assessment (HRA). Using the 40-mg/d dose and assuming oral dose administration to a 70-kg adult, the human no-observed-effect-level (NOEL) would be 0.571 mg/kg/d.
b. Dermal Exposure
Humans may be exposed directly to lindane through using medicinal preparations that are used to treat lice, scabies, and other similar skin parasites. Side effects from medicinal treatment occur mainly when the lindane treatment is misused or inappropriately prescribed.[963] However, there have been cases where neurotoxicity occurred despite proper treatment.
In a case of acute lindane poisoning, a 24-year-old woman suffered nonstop, uncontrolled body movements and visual hallucinations shortly after using lindane shampoo. The woman also was agitated, displayed constant athetotic writhing, involuntary movement, and multiple blisters over arms and legs and external genitalia. The woman had used two successive treatments of lindane shampoo (2 oz followed by 1 oz) in the course of 1 hour. The concentration of lindane in the shampoos was not reported. The lindane serum level was 3 ppb 20 hours after the application.[964] Because of an inability to obtain a definite dose from this study, no comparison to the HRA estimated route-specific doses could be made.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the NIH-cited benchmark dose discussed above will be used for HRA comparison under the assumption of equivalent absorption in oral and inhalation routes.
2. Subchronic Health Effects
a. Oral Exposure
Effects from the ingestion of lindane can include nausea, vomiting, and such neurotoxic effects as grand mal seizures, visual hallucinations, movement disorders, and coma. Subchronic oral health effects tend to produce much the same response as subchronic dermal health effects.
The NIH-cited human study discussed in the acute oral section above was conducted over a 14-day period. Because this time frame borders between acute and subchronic definitions, this same benchmark dose will be used for acute and subchronic comparisons to the HRA estimated route-specific doses. The assumptions stated in relation to that study remain the same throughout this review.
b. Dermal Exposure
Common neurological effects of subchronic skin exposure to lindane are grand mal seizures, visual hallucinations, movement disorders, restlessness, irritability, confusion, nervousness, dizziness, and coma. Subchronic exposure may also cause defatting dermatitis that is associated with incorrect application of medicinal treatments. According to the National Toxicology Program (NTP), the lowest published toxic dermal dose for humans is 20 mg/kg (during a six-week period).[965] It is uncertain whether this is an absorbed or applied dose and, for this reason, the data from this case study will not be used in comparing HRA route-specific doses with published health effects.
In a case of subchronic lindane poisoning, a 24-year-old woman reported having itchy bumps and visual hallucinations. She also presented with a headache, restlessness, and anxiety. The woman had applied an unknown concentration of lindane over her entire body and left it on 8-12 hours for 24 consecutive days. The lindane blood serum concentration was 7 ppb.[966] Because of an inability to obtain a definite dose from this study, no comparison to the HRA estimated route-specific doses could be made.
c. Inhalation Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature. For this reason, the benchmark dose cited by NIH discussed above will be used for HRA comparison under the assumption of equivalent absorption in oral and inhalation routes.
3. Chronic Health Effects
In human chronic exposures to lindane, symptoms include many of the acute exposure health effects listed above, plus degenerative changes in the liver and renal tubules and reduced reproductive capacity in both sexes.[967] Lindane and other organochlorines can be associated with signs and symptoms such as apathy, headache, uncontrolled mood swings, depression, confusion, and irritability in chronic exposures.[968]
One case of chronic lindane poisoning reports of a 45-year old male suffering fleeting episodes of blurred vision that became persistent after 3 months; he then developed occipital headaches and tinnitus. He was diagnosed with chronic intracranial hypertension that resulted from chronic use of lindane. The man had used lindane at least twice a month for 30 years to treat his hunting dogs for fleas and ticks. He used a 20 percent concentrate for spraying and dipping and claimed that he used a mask and proper clothing when applying the pesticide.[969]
In a study of 15 women exposed to wood-preserving chemicals in their homes for an average exposure period of 10 years, researchers found that the mean lindane serum blood levels were 0.085 µg/L (lindane serum levels in unexposed women were 0.043 µg/L). The women exposed to the lindane had subjective complaints and showed an altered neurobehavioral performance through standardized testing.[970]
4. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Tab D, Section D.3.a.(2), "Other Human Benchmarks."
Table 161. Lindane, comparison of HRA doses to benchmarks (application exposure)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based on Table 161, it seems the HRA route-specific dose estimates are below the range where human health effects were reported in the literature. To illustrate this, a comparison of doses in a high subchronic oral exposure scenario shows that the dose at which no human health effects were observed is 7.3 times the HRA estimated dose. When comparing high subchronic inhalation exposures, the dose at which no human health effects occur is 1.3 times above the HRA estimated dose (Figure 27)
5. Uncertainty/Variability of this Comparative Risk Characterization
Because reportable dose response data are lacking, there is significant uncertainty with this risk characterization. As mentioned above, the benchmark dose used in the comparison is based on assumptions of exposure route, absorption equivalency between exposure routes, and uncertainty in the definition of "no ill effects." Further uncertainty arises because of insufficient information about the human test group.
For these reasons, caution should be exercised when drawing conclusions from these data.
6. Risk Communication Summary
Based on published benchmarks,
the HRA calculated dose estimates seem to be well below doses at which human
health effects occur. It should be noted, however, that this is based on the
assumptions communicated throughout this text and a statistically significant
comparison is not possible because of a lack of sufficient dose response data.
Figure 27: Representation of Estimated Risk
Comparative representation of published human health risk to the HRA route-specific dose estimates. The above graph represents the lowest concentration of Lindane at which human health effects occur in the literature compared to the HRA estimated exposure of Gulf War servicemembers. The arrow represents the most conservative NOEL for all inhalation exposures from the human health effects studies. This level can then be compared to the exposure route that had the highest reported HRA dose estimate for all exposure levels.