A. Acute Health Effects
CIBA-GEIGY (now Novartis) has stated that human health effects from experiences specific to humans from mild to moderately severe poisoning include headache, weakness, sweating, indisposition, vomiting, nervousness, and difficulty swallowing. A severe exposure may result in profuse sweating, eye pain, miosis, muscular twitching, slurred speech, hypersalivation, respiratory distress, colic, heart complaints, convulsions, and unconsciousness. No dosages were reported for these human health effects. [807]
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
According to CIBA-GEIGY, azamethiphos may cause sensitization through skin contact.[808] Health effects for acute dermal exposure may be similar to the health effects for acute oral exposure.
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Health effects for acute inhalation exposure may be similar to the health effects for acute oral and dermal exposure.
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
B. Subchronic Health Effects
Although little exposure data are available for azamethiphos, subchronic exposure health effects for this cholinesterase (ChE) inhibitor may be similar to the effects of an acute exposure.
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
Little data are available on chronic exposure to azamethiphos. Health effects for a chronic exposure may be similar to the effects of an acute or subchronic exposure.
Human health effects studies providing toxicology benchmark dosages for chronic oral, dermal, and inhalation exposure routes were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data.
Because of a lack of dose response data for human health effects from azamethiphos exposure, we could not make a comparison between the Health Risk Assessment (HRA) doses and any peer-reviewed studies.
Table 144. Azamethiphos, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| Azamethiphos 1% crystals |
Low | A |
— |
— |
1.16E–02 |
— |
— |
— |
6.71E–05 |
— |
| Med. | S |
— |
— |
2.17E–02 |
— |
— |
— |
1.34E–04 |
— |
|
| High | C |
7.14E–03 |
— |
— |
— |
1.48E–02 |
— |
2.69E–04 |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic | |
| a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose |
Table 145. Azamethiphos, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDa HRA |
PDRDa |
ADDa HRA |
ADDa Literature |
HRA |
Literature |
|||
| Azamethiphos 1% crystals | Low | — |
— |
— |
— |
— |
— |
— |
— |
— |
| Med. | S |
4.29E–06 |
— |
— |
— |
— |
— |
— |
— |
|
| High | C |
1.55E–03 |
— |
— |
— |
— |
— |
— |
— |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic | |
| a PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose |
E. Uncertainty/Variability of this Comparative Risk Characterization
Because of a lack of health effects data on human exposure to azamethiphos, we could not make a comparative risk evaluation.
F. Risk Communication Summary
Because of a lack of health effects data on human exposure to azamethiphos, caution should be exercised when using this pesticide until epidemiological and toxicological studies become available.
VI. METHOMYL
A. Acute Health Effects
Methomyl is a carbamate pesticide initiating cholinesterase (ChE) inhibition. The acute toxic action of methomyl is characterized by signs of anticholinesterase action such as lacrimation, profuse salivation, tremor, and pupil constriction. Methomyl has a well-known mechanism of toxic action with particular toxicity by the acute oral and inhalation routes. As with other carbamate pesticide active ingredients, methomyl is excreted rapidly and does not accumulate in mammalian tissue. If exposure is suspended, ChE inhibition and its symptoms rapidly reverse.
1. Oral Exposure
Accidental and suicidal poisonings provide the bulk of human exposure data for methomyl. One instance of accidental poisoning comes from five Jamaican fishermen who added methomyl to their meal instead of salt. Methomyl had been stored in an unlabeled tin can and was mistakenly used in preparing "roti," an Indian dish. Only minutes passed before three of the men began twitching, trembling, and frothing at the mouth. All died within 3 hours of ingestion. Estimations from food samples show that the individuals consumed between 12 mg/kg and 15 mg/kg methomyl, which is considered a lethal dose.[809]
In nonfatal cases, illness generally lasts less than 24 hours. Based on qualitative and quantitative methomyl toxicity characteristics, 0.03 mg/kg/d is unlikely to cause adverse effects in humans by any exposure route. Accidental and intentional human poisoning data identify similar acute levels of methomyl toxicity in humans to that found in laboratory animals.[810]
The deaths of a 31-year old woman and her son concur with the previously cited lethal methomyl dose. Examination after their deaths identified 15.4 mg/kg methomyl concentrations. The estimated doses for the son and his mother were 13 mg/kg and 55 mg/kg, respectively.[811]
A 79-year-old man and his 73-year-old wife attempted double suicide by ingesting methomyl powder. The woman died 19 hours after ingestion despite intensive care. An autopsy revealed a large number of miliary hemorrhages found in both thalami of the brain. Her husband recovered after 10 days of treatment. Analysis revealed the methomyl concentration was 44 �g/g in the wife’s serum sample collected 1 hour after ingestion, and 0.2 �g/g in the blood sample collected at autopsy. The methomyl concentration in the husband’s blood sample collected 28 hours after ingestion was 0.01–0.1 �g/g. An estimated 11 g methomyl were ingested between the 2 of them. Methomyl powder, weighing approximately 5 g, amounts to approximately 30 mg/kg.[812]
2. Dermal Exposure
Because of the slower absorption phase of methomyl, acute dermal toxicity is low. Acute dermal exposures allow for recovery time from toxic action; therefore, the effect is never fully exerted.[813]
3. Inhalation Exposure
A health surveillance study in 22 healthy spraymen showed significant T-wave changes (including inversion) following a 5-day exposure to methomyl. Significant changes in plasma ChE and lactic dehydrogenase activities were also noticed. This is the first reported type of change in occupationally exposed subjects following exposure to a carbamate compound. Study results indicate that these changes are probably related directly to methomyl rather than its toxicity through ChE inhibition. The study identifies significance of these changes and the need for further investigation.[814]
In an unpublished document, more than 225 poisonings were reported between 1972 and 1973 following inhalation exposure to dry formulations of methomyl. Some exposure incidences resulted in serious acute reactions, but none were fatal. After this compound was reformulated as a liquid, cases decreased to fewer than 10 per year.[815]
In another case, methomyl was found in the blood of a pilot who died when his aircraft crashed while he was spraying methomyl. Circumstances of the pilot’s case suggest that the methomyl poisoning probably occurred by inhalation and dermal absorption. The pesticide active ingredients in his blood were measured by gas chromatography with flame photometric detection and the results confirmed by mass spectrometry with direct liquid injection through a liquid chromatography interface. The whole blood methomyl concentration was 570 ng/mL.[816]
Specific dosage-related acute human inhalation studies were not found in the research literature. For this reason, data from the acute oral human studies will be used for making comparisons to the Health Risk Assessment (HRA) exposure estimates.
B. Subchronic Health Effects
1. Oral Exposure
Human health effects studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
C. Chronic Health Effects
1. Oral Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
2. Dermal Exposure
Human health effect studies providing toxicology benchmark dosages for this exposure route were not found in the research literature.
3. Inhalation Exposure
One study identified chronic inhalation exposure in a plant manufacturing methomyl. The workers’ average employment duration was 2 years. Eleven workers were hospitalized for illnesses related to methomyl exposures. The highest hospitalization rates occurred in areas involved with chemical packaging (27 percent), production (22 percent), and plant maintenance (9 percent). Clinical evaluation revealed 46 percent of the 11 packaging workers experienced the highest frequency of acute cholinergic symptoms, including miosis, blurred vision, nausea or vomiting, muscle weakness, fatigue, and increased salivation. No precise dose response or chronic health effects were determined and reliable ChE measurements were not made.[817]
Human health effects studies providing specific toxicology benchmark dosages for this exposure route were not found in the research literature.
D. Risk Characterization: Comparison of HRA Modeled Dose Estimates to Non-Carcinogenic Health Effects
Please refer to the reference table(s) in Section B.4.C.1.b of Other Toxicity Benchmarks from Human Data..
Table 146. Methomyl, comparison of HRA doses to benchmarks (application exposure)
Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDf HRA |
PDRDf Literature |
ADDf HRA |
ADDf Literature |
HRA |
Literature |
|||
Methomyl |
Low |
A |
— |
3.0E–02a |
1.16E–02 |
3.0E–02b |
— |
— |
6.71E–05 |
3.0E–02b |
Med. |
S |
— |
3.0E–02e |
2.17E–02 |
3.0E–02e |
— |
— |
1.34E–04 |
3.0E–02e |
|
High |
C |
7.14E–03 |
3.0E–02e |
— |
3.0E–02e |
1.48E–02 |
3.0E–02e |
2.69E–04 |
3.0E–02e |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a Lowest-observed-effect-level (LOEL) |
| b Based on acute oral data; assumes equivalent absorption. |
| c No-observed-effect-level (NOEL) data based on 21-day oral exposure study |
| d No chronic data are available. This data point is based on the 21-day subchronic data. |
| e No subchronic or chronic data are available. This is acute data from a single oral dose. |
| f PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
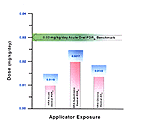
The HRA/Literature comparison table for application exposure identifies HRA calculated route-specific doses below those identified in the literature. If equivalent absorption is assumed for both oral and inhalation routes, the HRA estimated dose for acute inhalation of methomyl is approximately 2.0E-03 times the level where health effects were observed in the literature. Differences in dermal exposure levels are less significant. [Figure 19]
Table 147. Methomyl, comparison of HRA doses to benchmarks (post-application exposure)
Post-Application Exposure |
||||||||||
Pesticide |
Exp. Level |
Type |
Route-Specific Dose (mg/kg/d) |
|||||||
Oral |
Dermal |
Inhalation |
||||||||
HRA |
Literature |
PDRDf HRA |
PDRD Literature |
ADDf HRA |
ADD Literature |
HRA |
Literature |
|||
Methomyl
|
Low |
— |
— |
3.0E–02a |
— |
3.0E–02b |
— |
— |
— |
3.0E–02b |
Med. |
S |
4.92E–06 |
3.0E–02e |
— |
3.0E–02e |
— |
— |
— |
3.0E–02e |
|
High |
S |
1.55E–03 |
3.0E–02e |
— |
3.0E–02e |
— |
3.0E–02e |
3.47E–02 |
3.0E–02e |
|
| * Exposure type: A = acute/subacute; S = subchronic; C = chronic |
| a Lowest-observed-effect-level (LOEL) |
| b Based on acute oral data; assumes equivalent absorption. |
| c No-observed-effect-level (NOEL) data based on 21-day oral exposure study |
| d No chronic data are available. This data point is based on the 21-day subchronic data. |
| e No subchronic or chronic data are available. This is acute data from a single oral dose. |
| f PDRD = potential dose rate for dermal contact; ADD = absorbed dermal dose. |
A similar pattern appears in the post-application exposure HRA/Literature table. Data from the HRA enable comparisons with the chronic inhalation, subchronic oral, and chronic oral routes. The greatest estimated dose of 3.47E–02 mg/kg/d listed under chronic inhalation is slightly above the literature-extrapolated dose at which human health effects occurred.
E. Uncertainty/Variability of this Comparative Risk Characterization
Because of a limited amount of documented human dose response and occupational exposure/health effects data, there is significant uncertainty with this risk characterization. Minimal human data identified from the literature necessitate extrapolated comparisons of subchronic and chronic health effects from acute doses and assumptions of equivalent route-specific absorption. In addition, there is uncertainty because of limited data about identified human test groups.
For these reasons, caution should be exercised when drawing conclusions from these data.
F. Risk Communication Summary
Based on limited published human health benchmarks for methomyl, the HRA calculated dose estimates seem to fall below doses where identified human health effects occur. It should be noted, however, that this is based on the assumptions communicated throughout this text and a statistically significant comparison is not possible because of a lack of sufficient dose response data.
Figure 19. Representation of Estimated Risk
| First Page | Prev Page | Next Page |